Aortic stenosis (AS) is one of the most common valvular heart diseases, characterised by narrowing of the aortic valve orifice, leading to obstruction of blood flow from the left ventricle to the aorta. The primary cause of AS is typically degenerative calcification of the aortic valve (Figure 1), which commonly occurs in elderly individuals. However, other factors, such as congenital bicuspid aortic valve (Figure 2) in relatively young individuals, rheumatic heart disease and radiation-induced injury, can also contribute to the development of AS.1 With the ageing population, the prevalence of AS is expected to increase in the community. The Australian prevalence of severe AS is estimated at 1.5% of the population aged >55 years and 3.5% in those aged >75 years, with a yearly incidence of 1.8 per 1000 people.2
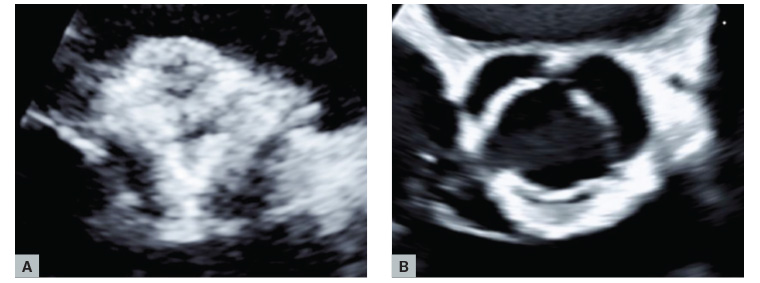
Figure 1. Echocardiography showing (A) a heavily calcified aortic valve and (B) a normal aortic valve.
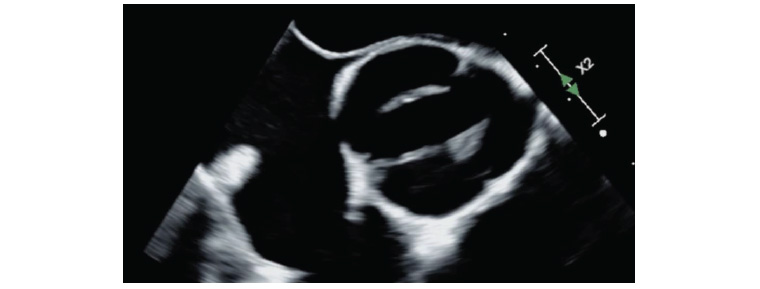
Figure 2. Transoesophageal echocardiography showing a cross-section of a bicuspid aortic valve in systole. Image courtesy of Dr Laila Khan.
Early detection and management are crucial for optimal patient outcomes in severe AS because untreated AS is associated with a two-year mortality rate of 30–50%.1 The severity assessment of AS involves evaluating clinical symptoms and conducting an echocardiographic assessment of valve parameters, including aortic valve area, velocity and pressure gradient, as well as computed tomography calcium scoring. The treatment of AS has traditionally centred around surgical aortic valve replacement (SAVR). However, with the advent of transcatheter aortic valve implantation (TAVI) as a newer treatment option, the landscape is undergoing a transformation. TAVI offers an alternative for patients who were previously deemed unsuitable for surgery, but its implementation requires a more complex and multidisciplinary approach.
Clinical features and assessment of AS
During routine examinations in asymptomatic patients, the presence of a systolic murmur (Table 1) often leads to the suspicion of AS within the community. Exertional dyspnoea or decreased exercise tolerance is the most common initial symptom. Dyspnoea, symptoms of heart failure, angina and syncope are considered the cardinal symptoms of AS.3
| Table 1. Differential diagnosis of a systolic murmur16 |
| Valvular heart disease |
Site and radiation |
Accentuation and dynamic manoeuvres |
Other features |
| Aortic stenosis |
An ejection systolic (mid-systolic) murmur in the right second intercostal space, typically radiating to the neck |
Murmur becomes softer on hand grip due to increased afterload |
- Low-volume pulse
- Systolic thrill
- Reduced pulse pressure
- Apex is generally not displaced
- Soft second heart sound
- ECG frequently shows features of LVH
|
| Hypertrophic cardiomyopathy |
Late systolic murmur along the left lower sternal border and pansystolic murmur due to associated mitral regurgitation |
Due to decreased venous return, murmur becomes louder with Valsalva manoeuvres and on standing
Murmur decreased by squatting |
- Sharp, jerky carotid pulse
- Double apical impulse
- Prominent ‘a’ waves in the JVP
|
| Mitral regurgitation |
Pansystolic murmur maximal at the apex that radiates to the axilla
Late systolic murmur in the presence of mitral valve prolapse |
Hand grip makes the murmur louder |
- Apex beat is displaced downwards and outwards, forceful in character
- Soft first heart sound
- Presence of third heart sound is common
|
| Tricuspid regurgitation |
Localised, pansystolic murmur over the left lower sternal border |
Increases in inspiration |
|
| Pulmonary stenosis |
Ejection systolic murmur in the second left intercostal area |
Best heard on inspiration |
- Giant ‘a’ waves in the JVP
- Absence of radiation to the neck
|
| Ventricular septal defect |
Loud pansystolic murmur at the left lower sternal area
Localised |
The murmur is louder on expiration |
- Thrill at the left sternal edge
|
| ECG, electrocardiogram; JVP, jugular venous pressure; LVH, left ventricular hypertrophy. |
As AS progresses, it can lead to increased myocardial oxygen requirements due to progressive left ventricular hypertrophy. This hypertrophy might also compress the intramural coronary arteries as they transport blood to the myocardium. In addition, reduced diastolic filling of the coronary arteries can occur, which can result in angina, even in the absence of coronary artery disease.4 When AS is severe, cardiac output does not increase as expected during exercise. This can cause a drop in systemic vascular resistance during exertion, potentially leading to hypotension and syncope.5
When evaluating a patient with a known or suspected AS, it is important to begin with a thorough history and physical examination, with a specific focus on the cardiovascular system, to identify a typical ejection systolic murmur (Table 1). In addition, an electrocardiogram should be performed to assess rhythm and the presence of left ventricular hypertrophy (Figure 3). A chest X-ray is useful to evaluate heart size, the presence or absence of pulmonary congestion or other pathology.
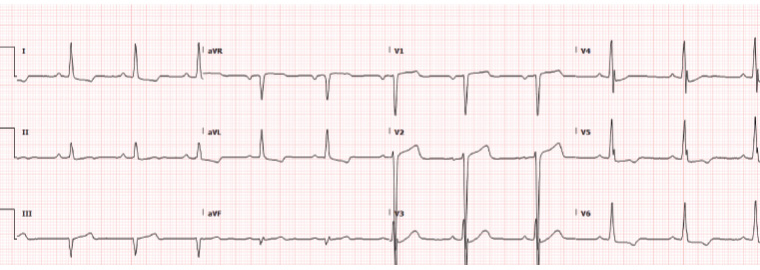
Figure 3. Electrocardiogram showing normal sinus rhythm with left ventricular hypertrophy as evidenced by deep S waves in V2, a tall R wave in V5 and ST-T segment abnormalities in Leads I, aVL and V6.
Although a detailed physical examination can be helpful, it might not always accurately diagnose or assess the severity of AS.
For patients with suspected AS, transthoracic echocardiography is the recommended initial test. Echocardiography helps accurate identification of the number of valve leaflets, assessment of valve motion (Figure 1B, Figure 2), the presence and extent of calcification (Figure 1A), associated other structural heart disease and left ventricular function.
In the echocardiographic evaluation of patients with AS, four broad categories can be defined, as listed below.6
- High-gradient AS (mean gradient ≥40 mmHg, peak velocity ≥4.0 m/s, valve area ≤1 cm2 [or ≤0.6 cm2/m2]). Although echocardiographic parameters in severe AS typically show high flow and a high gradient (across the valve), a low-flow state might occur with associated reduced left ventricular systolic function (classical) or, at times, with a preserved function (paradoxical low flow) and it is often associated with a low transvalvular gradient given that the gradient is highly flow-dependent. In a low-flow state, the gradient might be pseudo-normalised and thus underestimate the stenosis severity, whereas the aortic valve area might be pseudo-severe and thus overestimate the severity.7
- Low-flow, low-gradient AS with reduced ejection fraction (mean gradient <40 mmHg, valve area ≤1 cm2, left ventricular ejection fraction [LVEF] <50%, stroke volume index [SVi] ≤35 mL/m2). Low-dose dobutamine stress echocardiography (DSE) is recommended to distinguish between true severe and pseudo-severe AS.6
- Low-flow, low-gradient AS with preserved ejection fraction (mean gradient <40 mmHg, valve area ≤1 cm2, LVEF ≥50%, SVi ≤35 mL/m2). In these patients, the low-flow state is related to a concentric remodelling with impaired left ventricular filling, leading to low stroke volume, typically encountered in hypertensive elderly subjects with small left ventricle size and marked hypertrophy. The degree of aortic valve calcification (Figure 1) is a strong predictor of clinical outcome in AS. Quantitation of aortic valve calcium by computed tomography imaging is useful to corroborate stenosis severity when DSE is not feasible or not conclusive. The cut-off values of sex-specific Agatston aortic valve calcium score to identify true severe AS and predict mortality in women and men are ≥1200 and ≥2000 AU, respectively.8
- Normal-flow, low-gradient AS with preserved ejection fraction (mean gradient <40 mmHg, valve area ≤1 cm2, LVEF ≥50%, SVi >35 mL/m2). These patients usually have only moderate AS.6
Asymptomatic severe AS
Asymptomatic patients with severe AS and normal left ventricular systolic function have a similar survival rate to age-matched controls during the asymptomatic phase, with a low risk of sudden death (<1% per year).3 In the majority of cases, it is recommended that asymptomatic patients with severe AS undergo watchful waiting with repeat echocardiogram every 6–12 months.1 For mild disease, an echocardiogram at three to five years is recommended, increasing to one to two years for those with moderate disease.1
Exercise testing, performed by an experienced operator in a supportive setting, might provide important diagnostic and prognostic information in patients with severe AS who are asymptomatic. It helps to evaluate the haemodynamic effects of exercise and to verify the absence of symptoms.9
In symptomatic patients with severe AS, exercise testing should not be performed because of the risk of adverse outcomes.
Management
Aortic valve replacement is the only treatment that improves mortality in patients with symptomatic severe AS.6 Appropriate treatment at the onset of mild symptoms can prevent the development of heart failure, syncope or angina. For patients with symptomatic severe high-gradient AS, aortic valve replacement improves survival, symptoms and left ventricular systolic function.3
Symptomatic patients who have severe high-gradient AS with a mean gradient >40 mmHg, peak velocity >4.0 m/s and valve area <1.0 cm2 or <0.6 cm2/m2 should be evaluated for aortic valve replacement.6
Treatment of severe AS with either a transcatheter or surgical valve prosthesis should be based primarily on symptoms or reduced ventricular systolic function. In addition to clinical symptoms and examination findings, several risk scores, such as the EuroSCORE and Society of Thoracic Surgeons (STS) score, can aid in predicting outcomes and guiding treatment decisions in patients with AS.1 Earlier intervention may be considered if indicated by results of exercise testing, biomarkers, rapid progression or the presence of very severe stenosis.1
Although TAVI is often preferred for older patients (aged >75 years) or those at high risk of perioperative mortality, the decision between surgical and transcatheter intervention is ultimately made based on a comprehensive evaluation of clinical, anatomical and procedural factors.6 This evaluation is typically conducted through a discussion among the cardiologist, cardiothoracic surgeon, geriatricians and other relevant medical professionals in a ‘heart team’ meeting. For patients who undergo TAVI and have no pre-existing indication for oral anticoagulation, lifelong single antiplatelet therapy is recommended.10
Appropriate treatment of hypertension and hyperlipidaemia should be initiated for patients with AS. Approximately 40% of patients with AS also have hypertension.3
Antihypertensive treatment should be initiated at low doses and gradually up-titrated. Angiotensin-converting enzyme inhibitors are safe, well tolerated, improve haemodynamic parameters, augment effort tolerance and reduce dyspnoea in symptomatic patients with severe AS.11–13
Perioperative management of AS before non-cardiac surgery
AS increases the risk of perioperative complications during non-cardiac surgery (NCS).13 The level of risk associated with NCS depends on several factors, including the severity of stenosis, the presence of symptoms and the presence of co-existing cardiac disease.
Severe symptomatic AS is a significant risk factor for postoperative myocardial infarction and heart failure, and is a predictor for mortality after NCS.14,15 For symptomatic patients with severe AS who are scheduled for elective intermediate- or high-risk NCS, aortic valve replacement is recommended.15
However, asymptomatic patients with severe AS and normal LVEF can safely undergo low- to intermediate-risk NCS if the procedure is not associated with large volume shifts.15
In addition, antibiotic prophylaxis should be considered for high-risk procedures in patients with prosthetic valves, including transcatheter valves or repairs involving prosthetic material, as well as for patients with a history of infective endocarditis.
Conclusion
AS is a commonly encountered valvular heart disease characterised by the triad of angina, syncope and heart failure, along with examination findings of an ejection systolic murmur. Early recognition, assessment and referral can avoid significant morbidity and mortality. Echocardiography remains the cornerstone for confirming the diagnosis and determining the severity of AS, whereas exercise testing and risk scores might provide valuable prognostic information.
In the community, it is important to follow up with patients who initially have asymptomatic AS and promptly refer them when symptoms arise. Intervention, whether through surgical or transcatheter aortic valve replacement, is crucial for achieving optimal outcomes in AS patients. Coordinated care through multidisciplinary teams, including cardiologists, surgeons and general practitioners, is essential in current practice.
Key points
- Aortic stenosis (AS) is commonly encountered with high mortality in symptomatic patients if untreated.
- Clinical recognition of classic symptoms and signs of AS remains important.
- Echocardiography remains the most useful test to diagnose and stratify AS severity.
- The general practitioner can provide valuable serial assessment for symptom presence/progression.
- Suitability for surgical versus transcatheter valve replacement depends on individual patient factors.