Cardiac implantable electronic devices (CIEDs) have existed for over 50 years and continue to be an essential and reliable treatment for heart rhythm disorders.1 The major recent progress in CIEDs has been in their scope, functionality and individualisation. Treatment options now include pacemakers for the treatment of bradycardia (Figure 1); implantable cardioverter-defibrillators (ICDs) for the treatment of ventricular arrhythmias; cardiac resynchronisation therapy (CRT), a special form of pacemaker stimulating the left ventricle in patients with heart failure and reduced ejection fraction, which might include a defibrillator (CRT-D; Figure 2) or pacemaker only (CRT-P); and implantable loop recorders (ILRs), which are used to monitor and diagnose heart rhythm disorders where non-invasive methods, such as Holter monitoring, have been exhausted (eg infrequent, undifferentiated syncope).
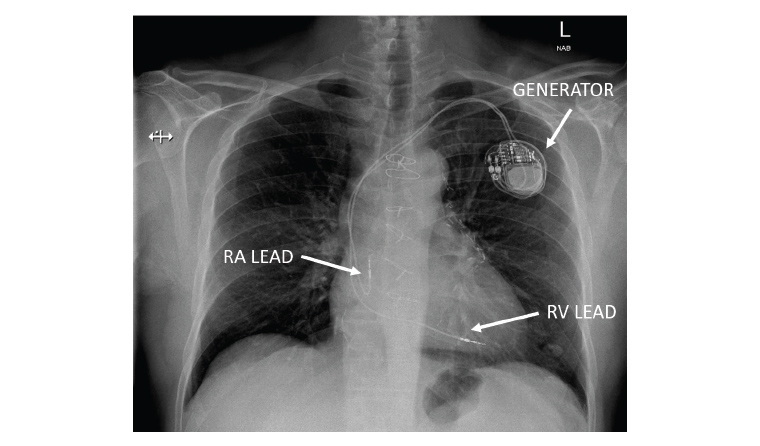
Figure 1. Chest X-ray (posteroanterior view) showing the typical components of a dual-chamber pacemaker system.
RA, right atrium; RV, right ventricle.
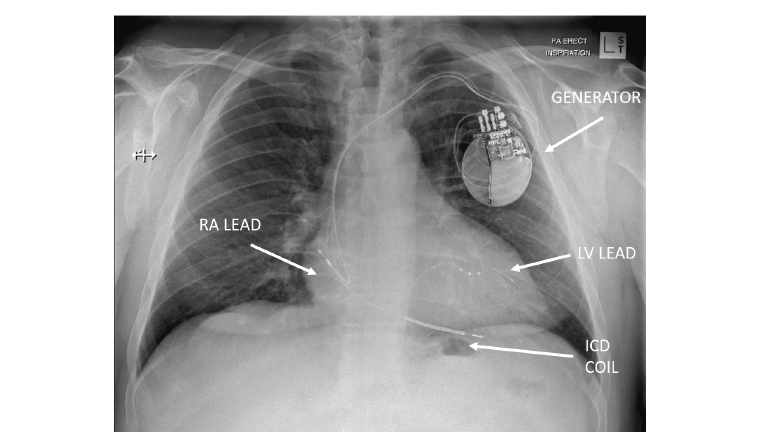
Figure 2. Chest X-ray (posteroanterior view) showing a biventricular pacemaker and defibrillator system (CRT-D).
ICD, implantable cardioverter-defibrillator; LV, left ventricle; RA, right atrium.
| Table 1. Indications for implantation of permanent pacemakers2 |
| Regardless of symptoms |
- Mobitz II second-degree, high-grade or third-degree AV block
|
| Symptomatic patients |
- Sinus node dysfunction
- Atrial fibrillation and bradycardia
- Bradycardia due to required medical therapy (eg beta-blockers in heart failure)
|
| Special circumstances |
- Mobitz I second-degree or marked first-degree AV block
- Refractory vasovagal syncope
- Long QT syndrome
|
| AV, atrioventricular. |
The indications for a permanent pacemaker implant (Table 1) and ICD implant (Table 2) remain relatively unchanged.2,3 Some special indications in populations with genetic disorders or cardiomyopathies might exist outside of this. Although the definition of sinus bradycardia is a heart rate <50 beats per minute, if no symptoms exist, slower rates might be observed without requiring permanent pacing.2 An overview of commonly used pacemaker mode terminology is included in Table 3.1
| Table 2. Indications for implantation of cardioverter-defibrillators3 |
| Secondary prevention |
- Survived cardiac arrest
- Sustained ventricular tachycardia >30 seconds
|
| Primary preventionA |
- LVEF <35% (NYHA Class II–III)
- LVEF <30% (NYHA Class I)
|
| Special populationsB |
- Hypertrophic cardiomyopathy
- Long QT syndrome
- Brugada syndrome
- Arrhythmogenic right ventricular cardiomyopathy
|
AOn guideline-indicated medical therapy, prognosis >12 months and 40 days after myocardial infarction/90 days after percutaneous coronary intervention/coronary artery bypass grafting.
BWhen indicated by disease-specific guidelines.3
LVEF, left ventricular ejection fraction; NYHA, New York Heart Association. |
| Table 3. Examples of pacemaker modesA |
| I |
II |
III |
IV |
| Chamber paced |
Chamber sensed |
Response to sensing |
Rate modulation |
| A = atrium |
A = atrium |
T = triggered |
R = rate adaptive |
| V = ventricle |
V = ventricle |
I = inhibited |
|
| D = dual |
D = dual |
D = dual |
|
| O = none |
O = none |
O = none |
O = none |
| Examples |
Notes |
| V |
V |
I |
Single-chamber ventricular pacing |
| D |
D |
D |
Dual-chamber pacing (most common) |
| D |
D |
I |
Dual-chamber pacing and sensing without tracking of atrial rate |
| V |
O |
O |
Asynchronous ventricular pacing without sensing underlying rhythm |
| AFor example, a device programmed VVIR 60–120 beats per minute (b.p.m.) will sense and pace in the ventricle only, at a minimum rate of 60 b.p.m., increasing progressively to a maximum rate of 120 b.p.m. when physical activity is detected.1 |
With the gathering speed of technological advances in electronics, wireless connectivity and computing power, the expansion in options to treat patients with CIEDs continues at pace. Developments include reduced size, increased battery longevity, leadless devices, enhanced diagnostic capabilities, remote monitoring capability and the addition of magnetic resonance imaging (MRI)-compatible devices.1
Leadless pacemakers
In response to limitations of traditional transvenous systems (ie infection of the pocket and/or indwelling leads, pneumothorax during implant and subclavian venous thrombosis/stenosis), efforts have been made to develop leadless cardiac pacing systems.4
These systems are designed with the pulse generator and electrode built into a small capsule with a fixation mechanism on the distal end of device that directly anchors into heart muscle (Figures 3,4).4 In general, leadless pacemakers are placed percutaneously, via the femoral vein, and implanted within the right ventricle.4
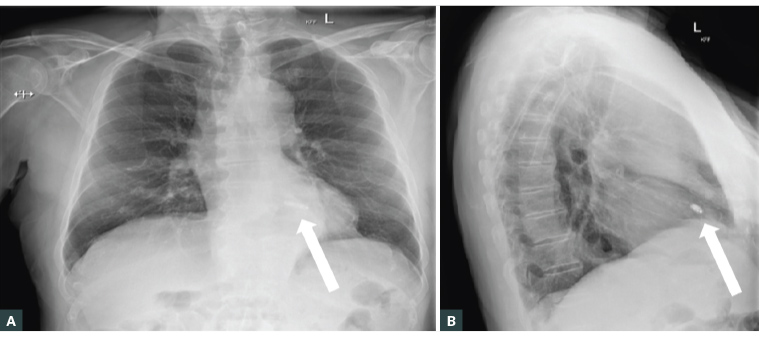
Figure 3. Chest X-ray showing posteroanterior (A) and lateral (B) images showing the typical location of a leadless pacemaker (arrows).
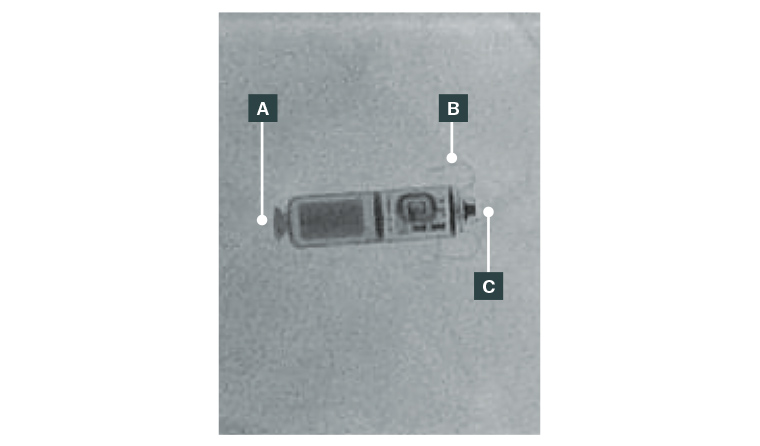
Figure 4. Magnified image of a leadless pacemaker: (A) Retrieval mechanism; (B) Fixation tines; (C) Cathode.
The original leadless pacemaker models were designated for single-chamber right ventricular pacing (pacing mode VVI, VVIR or VOO) without atrial sensing and hence are generally reserved for use in permanent atrial fibrillation.
Since 2021, atrial sensing (but only ventricular pacing) has been available in the leadless Micra AV device (Medtronic).5 This device has the ability to maintain AV synchrony using an accelerometer-based algorithm in patients with normal sinus node function and complete heart block by coordinating right ventricular pacing with sensed atrial contractions (pacing mode VDD).5 A true dual-chamber leadless system has recently debuted, but is not yet available in Australia (pacing mode DDD).6
Leadless pacing might be the future of the field, but some trade-offs do exist, including potential for femoral vascular injury, dislodgement, pericardial effusion and substantially higher cost. In addition, because battery life is anticipated to be approximately 10 years, careful consideration should be taken with routine use in younger patients as multiple devices across the course of a lifespan might obstruct flow into or out of the right ventricle.
Subcutaneous defibrillators
Concerns around infection and indwelling lead-related complications with pacemakers are also applicable to defibrillator leads.7
To reduce these complications, a subcutaneous form of ICD, implanted beneath the skin and external to the thoracic cavity, has been developed (Figure 5). Implantation involves tunnelling the ICD lead subcutaneously over the sternum and connecting to a pulse generator usually implanted laterally, submuscular and between the serratus anterior and latissimus dorsi. Subcutaneous ICDs provide similar shock efficacy in terminating ventricular tachycardia/ventricular fibrillation, but do not have the pacing capabilities of traditional ICDs.7 Rates of lead-related complications such as infection, dislodgement, pneumothorax and tamponade are lower in subcutaneous ICDs, but inappropriate ICD shocks, due to oversensing, are higher.7
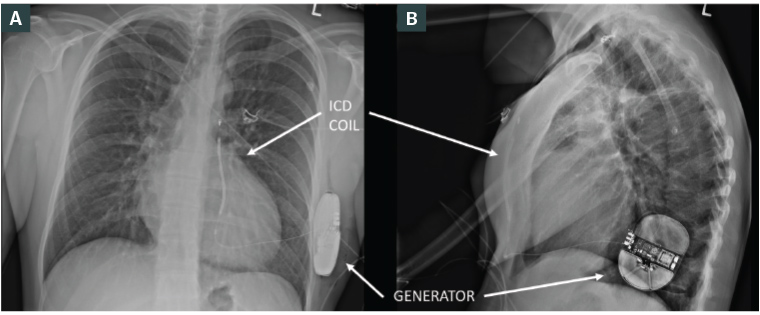
Figure 5. Chest X-ray showing posteroanterior (A) and lateral (B) images of a subcutaneous defibrillator.
ICD, implantable cardioverter-defibrillator.
MRI conditional pacemakers
MRI conditional pacemakers
It is estimated that 50–75% of patients with a cardiac device might need an MRI over their lifetime.8 Early safety concerns regarding MRI interaction with CIEDs causing potentially fatal loss of pacing, inappropriate ICD therapy or damage to leads led to an initial blanket contraindication to MRI.8
Given the increasing importance of MRI as a diagnostic tool, a significant amount of research was undertaken by pacemaker vendors to overcome safety concerns surrounding pacemaker patients undergoing an MRI. The result was several modifications to pacemaker systems, including minimising ferromagnetic content, replacement of reed switches, altering lead design (to reduce heat from electromagnetic energy), special pacemaker circuitry and dedicated pacemaker programming.9 This has led to the majority of contemporary CIEDs and lead systems being labelled ‘MRI conditional’ or ‘MRI compatible’ and, when predefined safety checks, management plans (including discussion with the radiologist and cardiologist) and conditions are followed, MRI can be performed safely.10
Conversely, there is still a significant number of patients who have older, non-conditional devices or abandoned leads (eg due to lead fracture) who might benefit significantly from an MRI. If an MRI is truly essential, there is evidence it can be performed safely at expert centres where appropriate electrophysiology expertise and imaging resources are available. 11,12
Remote monitoring
Conventional pacemaker follow-up required in-person device interrogation to assess system integrity, stored diagnostic information and battery longevity. Contemporary technology referred to as ‘remote monitoring’ has now enabled comprehensive and safe interrogation of most CIEDs without an in-person visit.13 Remote monitoring involves the transmission of data over a network from the patient’s location via a central server to a hospital or a physician’s office.14 To enable transmission from pacemaker to network, patients are given a remote monitor that is paired to the CIED. The device is manually interrogated by the patient using a telemetry wand built into the home monitor or, alternatively, via automatic wireless transmission depending on vendor and model of device.15,16 In more recent times, various vendors have developed technology that enables connectivity via mobile cellular networks and WiFi. Patients can download an application on their smartphone that connects to the CIED via Bluetooth technology.17
Clinical benefits of remote monitoring include the early detection of dangerous arrhythmias (allowing intervention that reduces mortality, as well as morbidity by reducing painful inappropriate defibrillator shocks) and detection of preclinical heart failure episodes (allowing intervention prior to hospital presentation). The patient experience is also improved with remote monitoring because routine checks can be performed remotely rather than at six- to 12-monthly visits to device clinics.
It should be noted that although data can be transmitted from CIEDs remotely, there is no ability to program devices remotely. Therefore, if programming changes are required, patients still need to attend the clinic in person.
Conduction system pacing
It has been established that, in some patients, traditional right ventricular apical pacing causes electric and mechanical desynchrony, and this is associated with an increased risk of atrial arrhythmias and heart failure.18 Conduction system pacing (CSP) is a technique that involves implantation of pacing leads within the native conduction system, including the His bundle and left bundle branch (LBB).19 The principle behind CSP is to activate the normal conduction system, which subsequently provides synchronised contraction of the ventricles, usually evidenced by a relatively narrow paced QRS complex (Figure 6).
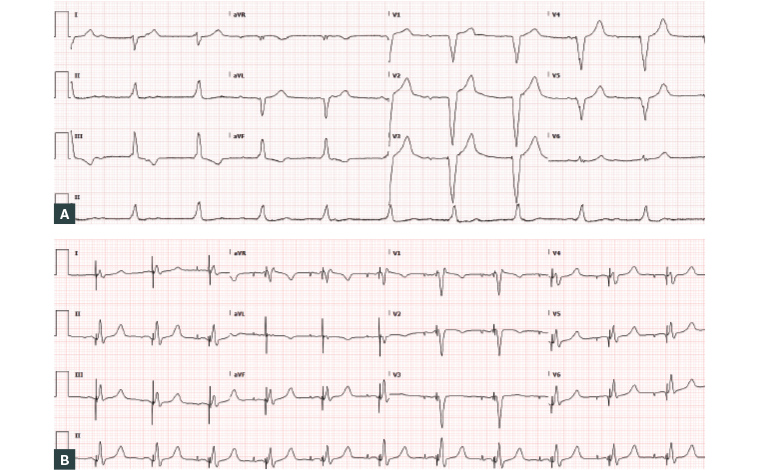
Figure 6. Electrocardiogram showing typical paced morphology from the right ventricular apex (A) compared with pacing from the left bundle branch area (B).
His bundle pacing (HBP) has been used as a form of CSP, but its widespread adoption has been limited by a challenging implant technique, unsatisfactory success rates in patients with underlying broad QRS, high pacing thresholds, low R waves, the potential to cause distal conduction block and early battery depletion.20
LBB area pacing (LBBAP) has now emerged as a reliable method to pace the conduction system.19 This procedure involves using specialised catheters to deliver the ventricular pacing lead deep into the interventricular septum to activate the left bundle.20 The advantages of LBBAP over HBP include a larger anatomical target site making the implant less technically challenging, more stable lead parameters and shorter procedure times with less use of radiation.19 Results from recent clinical trials demonstrate the feasibility and safety of LBBAP, but large randomised controlled trials are still ongoing.20–22
Pacemaker diagnostic information
One of the inadvertent benefits of having CIEDs with intracardiac electrodes has been the ability to obtain powerful diagnostic information. Since the first generation of pacemakers that were primarily designed to asynchronously pace the heart, there have been significant advancements in the diagnostic capabilities.
Contemporary pacemakers, depending on model and vendor, can record and store atrial and ventricular arrhythmias (including the date and time of an event), atrial fibrillation burden, heart rate histograms and heart rate variability. Devices can readily report the percentage of atrial and ventricular pacing, as well as trends of pacing lead performance. Measurement of intrathoracic impedance allows reporting of respiratory rate/minute ventilation, sleep apnoea episodes and heart failure episodes due to the changes in impedance that occur with fluid accumulation in the lungs.23,24 This same impedance-based mechanism can be used to detect and treat vasovagal syncope via algorithms that measure a drop in heart rate, or a relative change in pacing lead impedance, which occur at the onset of vasovagal episodes. Once a vagal episode is detected, the heart is then paced at a faster rate for a specified period to compensate for decrease in blood pressure and heart rate.25
Conclusion
CIED technology has progressed significantly in recent years and the latest advances in design, function and features have made CIEDs smaller, more reliable and more programmable. Advanced algorithms have improved the ability to detect and treat arrhythmias and other conditions, such as heart failure. The development of leadless and subcutaneous systems has led to more options for implantation and new features, such as remote monitoring and conduction system pacing, are now available. General practitioners should be aware of these advancements and the benefits they offer to patients with heart rhythm disorders.
Key points
- Permanent pacing continues to be a reliable treatment for bradycardias.
- Complexity is increasing in the scope of data available from cardiac devices.
- Most modern pacemakers are MRI compatible with appropriate oversight.
- Remote monitoring can provide rapid notification of lead or patient issues.
- Leadless pacemakers provide a new option in selected patients.