Cardiovascular diseases (CVDs) encompass a wide spectrum of disorders affecting the heart and blood vessels, including coronary artery disease, arrhythmias, heart failure and congenital heart defects. These conditions collectively contribute to a substantial global health burden. Historically, the understanding of CVDs focused on traditional risk factors such as hypertension, hyperlipidaemia and smoking; however, recent advances in genetics have uncovered the significant contribution of genetic factors in the pathogenesis of CVDs. General practitioners (GPs), as the frontline healthcare providers, can leverage this knowledge to improve risk assessment, diagnosis and provide personalised treatment strategies for their patients.
Aim
This article aims to summarise cardiac conditions that have a strong genetic basis while providing tools for GPs to address these conditions to improve health outcomes for the community.
Genetic basis of cardiac diseases
The understanding of genetics remains largely complex, and the interplay of genetic factors as well as the environment can never be undervalued. Advances in science have revealed the enormous amounts of variations between different individuals’ genetic code, which can sometimes raise more questions than answers.1
Determining whether a variant causes disease compared to one that is likely benign forms the cornerstone of cardiac genomics, and can be fraught with complexities, and is therefore best understood by clinical geneticists and specialists in the field. However, several principles stand true. A variant that is extremely rare, for example, is more likely associated with pathology than a variant that is found in a multitude of individuals. Furthermore, mutations within certain regions of a gene that are considered vital in coding for a particular protein’s function, are therefore more likely to be pathogenic. Meanwhile, understanding disease patterns in families can also expose disease-causing variants, by virtue of its presence in affected individuals only. This concept is known as co-segregation, and it requires phenotypic and genotypic information from multiple members of the same extended family, which can be difficult to establish (eg a small family with only one or two surviving family members). Perhaps the most precise way of determining pathogenicity is that of functional testing – whereby a variant of interest is genetically modified into an animal model, which then either physiologically or clinically exhibits a particular disease. These are, however, expensive, time-consuming and not readily available. The American College of Medical Genetics and Genomics classifies variants based on these principles, as well as with other methods such as in-silico testing, into those that are pathogenic (P) or likely pathogenic (LP) compared to benign variants, whereas several variants are considered unclassified (variant of unknown significance, VUS) due to a sparsity of data and evidence.
Regardless, the advances that we have experienced over the past 20 years have given us a better appreciation of familial diseases as well as one’s genetic predisposition to develop a CVD, which in turn might allow for tailored therapies based on an individual’s unique genetic code.
Monogenic cardiovascular disorders
A subset of CVDs arises from single-gene mutations with Mendelian inheritance patterns, resulting in predictable modes of transmission through families. From a cardiovascular viewpoint, these can be categorised into those that result in cardiomyopathies, arrhythmic diseases, vascular pathologies and lipid disorders, including familial hyperlipidaemia. The majority of these conditions are inherited in an autosomal dominant pattern, meaning that a first-degree family member of an affected individual has a 50% chance of being affected as well. Recognising these conditions early is crucial, as they often present with unique clinical features that warrant targeted interventions. Genetic testing can aid in identifying affected individuals and their at-risk family members.
Inherited cardiomyopathies
Inherited cardiomyopathies are a group of cardiac disorders characterised by structural and functional abnormalities of the myocardium, leading to cardiac dysfunction. Hypertrophic cardiomyopathy (HCM), dilated cardiomyopathy (DCM) and arrhythmogenic cardiomyopathy (ACM) represent significant subtypes with genetic underpinnings.
- Hypertrophic cardiomyopathy: HCM is primarily caused by mutations in genes encoding sarcomere proteins, such as MYH7, MYBPC3 and TNNT2. GPs should be aware of the clinical manifestations, including left ventricular hypertrophy, arrhythmias and sudden cardiac death (SCD). It remains the most common form of inherited cardiac diseases and is renowned for its incomplete penetrance, with one study showing that as many as 50% of clinically unaffected mutation carriers develop signs of HCM during the 15-year follow-up period.2 Genetic testing can aid in diagnosis, risk stratification and identification of at-risk family members for regular screening and genetic counselling,3 which is invaluable considering that sometimes the first presentation of the disease includes SCD.
- Dilated cardiomyopathy: DCM is characterised by left ventricular dilation and systolic dysfunction. Approximately 30–50% of DCM cases have a genetic basis, with mutations in genes encoding structural proteins, cytoskeletal components and ion channels implicated. Genetic testing can help identify familial cases, guide family screening and inform prognosis.4
- Arrhythmogenic cardiomyopathy: ACM is characterised by fibrofatty replacement of myocardium, leading to an increased risk of ventricular arrhythmias and SCD. Its classification has gone through considerable change over the past few decades, from description of the condition affecting the right ventricle predominantly (arrhythmogenic right ventricular cardiomyopathy), to then recognition of biventricular and left ventricular dominant forms.5 Mutations in desmosomal genes, such as PKP2, DSG2 and DSP, are commonly associated with ACM. Genetic testing and evaluation of family members are crucial due to the inheritable nature of this condition.6 Among the inherited cardiomyopathies, ACM progression can be linked to strenuous exercise.7 Identifying mutation carriers might allow for lifestyle changes that could alter the natural history of the condition, which once again underscores the importance of genomics in the tailoring of therapies.
Inherited channelopathies
Channelopathies are ion channel disorders that predispose individuals to abnormal cardiac electrophysiology and arrhythmias. The most common of these rare diseases include Long QT syndrome (LQTS), Brugada syndrome (BrS) and catecholaminergic polymorphic ventricular tachycardia (CPVT).
- Long QT Syndrome: LQTS is caused by mutations affecting ion channels responsible for cardiac repolarisation. It presents with prolonged QT intervals on electrocardiogram and can lead to life-threatening arrhythmias. Recognition of LQTS-associated symptoms (syncope, seizures, SCD) is vital, especially considering the dynamic nature of QT prolongation in this condition and therefore the occasional misdiagnosis. Genetic testing can identify affected individuals and guide risk stratification for tailored management and preventive measures.8 The genotype–phenotype correlations found in LQTS have been well studied, leading to treatment strategies targeted to the underlying molecular abnormality. These include the clear benefit of beta-blockade in LQTS type 1 whereby mutations in the KCNQ1 gene result in changes in the sympathetically sensitive, slowly activating delayed rectifier potassium channel (IKs), making antagonising the effects of catecholamines a successful therapy in this subtype.9 Current guidelines support these findings, removing some restrictions on certain types of competitive sports in LQTS type 1 sufferers who are compliant with their beta‑blocker regimes.10 As is well known, LQTS patients must avoid medications that might accentuate the abnormal repolarisation, including antihistamines, antibiotics and antidepressants, which in turn increases the risk of a life-threatening arrhythmia. Drugs that might potentiate these issues have been well researched and published.11
- Brugada syndrome: BrS is characterised by ST-segment abnormalities on electrocardiogram and an increased risk of ventricular arrhythmias, particularly in the right precordial leads. Genetic testing can identify mutations in sodium channel genes (SCN5A) and other genes associated with BrS. Early diagnosis and risk stratification are essential to prevent SCD. Once again, with increasing research into the pathophysiology of the disease, strategies such as treating pyrexia promptly, avoiding sodium channel blockers and managing excessive vagal tones are now recognised as important exogenous precipitants of malignant arrhythmias and therefore allow for mitigation in SCD risk.12
- Catecholaminergic polymorphic ventricular tachycardia: CPVT manifests as exercise-induced ventricular arrhythmias and syncope. Genetic testing for mutations in genes encoding cardiac calcium-handling proteins, such as RYR2 and CASQ2, is essential for diagnosis and management. Beta-blockers and implantable cardioverter-defibrillators are common preventive measures.13 Meanwhile, environmental situations that increase calcium overload such as with catecholamine surges in the context of thrill-seeking or high-level competitive sports, must be avoided.
Familial hypercholesterolaemia
Familial hypercholesterolaemia (FH) is an autosomal-dominant disorder characterised by high levels of low-density lipoprotein cholesterol (LDL-C) and an increased risk of premature cardiovascular disease. Mutations in genes involved in LDL receptor function and metabolism, such as LDLR, APOB and PCSK9, are implicated. GPs should be aware of clinical manifestations, perform lipid profiling, and consider genetic testing in suspected cases. Early identification and intervention can significantly reduce cardiovascular risk.13 Scoring systems, such as the Dutch Lipid Clinical Network Score (DLCNS) assist in identification of possible FH suffers, which might enable them to be treated with agents typically reserved for resistant hyperlipidaemia, such as the PCSK9 inhibitors.14 These monoclonal antibody treatments, which includes Evolocumab, are now available on the Australian Pharmaceutical Benefit Scheme when patients are already on a maximally tolerated statin and ezetimibe therapy but still have not achieved LDL targets.
Familial aortopathy
Familial aortopathy encompasses a spectrum of conditions such as Marfan syndrome, Loeys–Dietz syndrome and Ehlers–Danlos syndrome, among others. Mutations in genes encoding components of the extracellular matrix, particularly fibrillin-1 (FBN1), transforming growth factor-beta receptors (TGFBR1, TGFBR2) and collagen (COL3A1), have been identified as key contributors to these disorders.15 Affected individuals might present with a variety of clinical features, including aortic root dilation, dissections, aneurysms and connective tissue abnormalities. Non-aortic manifestations, such as ocular lens dislocation, joint hypermobility and skeletal abnormalities, can aid in differential diagnoses.16 Management of familial aortopathy primarily focuses on early detection, surveillance and preventive measures to reduce the risk of aortic complications. Regular cardiovascular evaluations, including imaging, blood pressure monitoring and genetic counselling, are essential components of management.17
Polygenic contributions
Polygenic traits, as opposed to monogenic traits, influenced by a single gene, arise from the cumulative effects of numerous genetic variants dispersed throughout the genome. Most CVDs result from a complex interplay of multiple genetic variants along with environmental factors. They exemplify a multifaceted polygenic architecture, where the collective influence of an array of genetic variants contributes to disease susceptibility. Genome-wide association studies (GWAS) have identified multiple genetic loci associated with CVD risk. These loci contribute to the heritability of complex cardiovascular traits, such as blood pressure, lipid levels and susceptibility to atherosclerosis. With GWAS, researchers have discovered a multitude of single nucleotide polymorphisms (SNPs) that have provided valuable insights into the intricate interplay of genetic factors in cardiovascular health. Although each individual variant might have a modest effect, their cumulative impact contributes to disease susceptibility.
Polygenic risk scores and cardiovascular diseases
Polygenic risk scores (PRS) are emerging as pivotal tools in assessing an individual’s genetic predisposition to complex traits, including CVDs. These scores amalgamate the cumulative effects of numerous genetic variants associated with CVDs, presenting a holistic perspective of an individual’s genetic risk landscape.
Benefits of PRS in CVD risk prediction include:
- Precision risk assessment: By amalgamating multiple genetic markers, PRS offer a refined risk assessment, enabling the identification of individuals at heightened risk who might otherwise be overlooked by traditional risk factors alone.
- Unravelling complex interactions: PRS highlight the interplay between numerous genetic variants, environmental factors and lifestyle choices that collectively shape an individual’s risk profile, contributing to a more comprehensive understanding of disease causality.
- Early intervention and personalised care: Timely identification of high-risk individuals via PRS empowers clinicians to implement targeted preventive measures and personalised treatment strategies, ultimately reducing the burden of CVDs.
Examples of where PRS have contributed to specific CVDs include:
- Coronary artery disease (CAD): The genetic underpinnings of CAD have been extensively studied, revealing several key insights. Khera et al conducted a ground-breaking study showcasing how a PRS can offer predictive power, even in individuals with seemingly low traditional risk factors.18 This suggests that genetic predisposition might play a more substantial role in CAD risk than previously appreciated.
- Atrial fibrillation (AF): AF, a prevalent cardiac arrhythmia, also exhibits polygenic influences. Roselli et al unveiled a PRS for AF that correlated with an elevated risk of developing the condition.19 These findings underscore the value of considering genetic markers alongside conventional risk factors for a more holistic assessment of AF susceptibility.
- Stroke: The genetic architecture of stroke, encompassing both common and rare variants, has been a focus of recent research efforts. Large-scale international consortia such as MEGASTROKE provide meta-analysis data of GWAS in stroke and have been instrumental in revealing the complex genetic landscape underlying different stroke subtypes.20 This work has paved the way for improved risk stratification and potential therapeutic targets.
Although the field of polygenic contributions to CVDs holds immense promise, challenges remain. First, genetic heterogeneity across populations and ethnicities poses challenges to creating universally applicable PRS for CVDs. Second, longitudinal validation in the form of long-term studies is needed to validate the predictive accuracy of PRS and assess their utility in guiding long-term management. Furthermore, there are several ethical considerations that remain integral to any genetic information, which includes responsible communication of genetic risk information and addressing potential psychosocial implications before PRS can be fully implemented. Currently, there are several commercial companies that perform PRS, but at present, its utility is clearly within the realm of research and hypothesis testing, rather than being clinically relevant. This, however, is likely to change in the years to come.
Pharmacogenetics
Pharmacogenetics involves the study of how an individual’s genetic makeup influences their response to medications. In the realm of cardiovascular disease, where drug interventions play a pivotal role in patient care, pharmacogenetic considerations hold immense promise. Variations in genes encoding drug-metabolising enzymes, drug transporters and drug targets can significantly impact drug efficacy, safety and dosing.
Clinical applications include:
- Antiplatelet therapy: Genetic variants such as CYP2C19 and its impact on clopidogrel metabolism have been well established. Incorporating genotyping into clinical decision making for antiplatelet therapy can help identify patients who are poor metabolisers of clopidogrel and might benefit from alternative medications.21
- Statins: Genetic variants in SLCO1B1 and PCSK9 genes influence statin response and metabolism. Utilising genetic information can aid in predicting statin efficacy and potential adverse effects, allowing for personalised dosing and drug selection.22
- Beta-blockers: Genetic variations in the beta-adrenergic receptor genes impact the response to beta-blockers in CVD patients.23 Tailoring beta-blocker therapy based on genetic profiles can optimise treatment outcomes.
- Warfarin: The VKORC1 and CYP2C9 genes are central to warfarin dosing. Pharmacogenetic testing helps determine optimal initial doses, minimising the risk of bleeding or inadequate anticoagulation.24
The integration of pharmacogenetics into CVD management offers the potential for enhanced treatment outcomes, reduced adverse events and improved patient satisfaction. By tailoring medications to individual genetic profiles, practitioners can optimise drug efficacy while minimising adverse effects. However, challenges such as accessibility to genetic testing, interpretation of results and limited clinical guidelines remain hurdles that need to be addressed. Consequently, the availability of genetic testing in pharmacogenetics is extremely limited and not currently mainstreamed.
Challenges and considerations: Implications for GPs
Since 2022, genetic testing for inherited cardiomyopathies and channelopathies in Australia is on the Medicare Benefit Scheme (MBS)25 if requested by a specialist or consultant physician, with most being performed in local laboratories rather than having samples sent overseas. Familial hypercholesterolaemia genetic testing has been on the MBS since May 2020, but there is still yet to be any rebates for testing familial aortopathies. The testing covers both proband testing as well as testing first- and second-degree family members. Testing guidelines are also available to physicians on when to perform these tests in relation to monogenic-inherited conditions, including cardiomyopathies, channelopathies, familial hyperlipidaemia and aortopathies.26,27
Indications for genetic testing for cardiovascular diseases
Overall, genetic testing is considered most appropriate in situations whereby a clinical diagnosis of an inherited CVD has been made. This is especially in situations whereby a family history of one of the aforementioned monogenic conditions is known, or there are strong suspicions of a genetic disorder; for example, if there is a family history of premature SCD (especially for those aged <40 years). In some conditions, genetic testing can also be part of the diagnostic process (such as in ACM or LQTS) rather than an adjunct in treatment (eg for predictive testing of at-risk family members or to guide management). Ideally, the patient who has evidence of the disease should be tested and is typically considered the index patient or proband. This maximises the chance of a positive test, considering with current knowledge, the yield of a positive result varies from disease to disease (Table 1). In certain circumstances, the index patient might present with sudden death whereby molecular autopsies can be ordered and genetic testing performed on stored DNA, with consent from the next-of-kin or Power of Attorney.
| Table 1. Gene panels, detection rates and clinical utility of genetic testing17,27 |
| CVD |
Common genes testedA |
Detection rate |
Clinical utility of the genetic test |
| Diagnostic |
Prognostic |
Therapeutic |
| HCM |
MYH7, MYBPC, TNNT2, TNNI3, TPM1, MYL2, MYL3, ACTC1 |
40–60% |
♥ |
♥ |
♥ |
| DCM |
ABCC9, ACTC, ACTN2, ANKRD1, CSRP3, CTF1, DES, DSG2, DSP, EMD, LDB3, LMNA, MYBPC3, PLN, SCN5A, SGCD, TAX, TCAP, TNNC1, TNNT2, TNNI3 TPM1, VCL |
<30% |
♥ |
♥ |
♥ |
| ACM |
DSP, DSG2, DSC2, PKP2 |
30–40% |
♥ |
♥ |
♥ |
| LQTS |
KCNQ1, KCNH2, SCN5A, KCNE1, KCNE2, KCNJ2 |
75–80% |
♥ |
♥ |
♥ |
| BrS |
SCN5A |
20–25% |
♥ |
♥ |
♥ |
| CPVT |
RYR2, CASQ2 |
50–60% |
♥ |
♥ |
♥ |
| FH |
LDLR, ApoB, PCSK9 |
25–75% |
♥ |
♥ |
♥ |
| Aortopathy |
FBN1, TGFBR1, TGFBR2, COL3A1 |
25% |
♥ |
♥ |
♥ |
| AF |
PRS |
Unreported |
♥ |
♥ |
♥ |
| CAD |
PRS |
Unreported |
♥ |
♥ |
♥ |
AList is illustrative and not exhaustive.
ACM, arrhythmogenic cardiomyopathy; AF, atrial fibrillation; BrS, Brugada syndrome; CAD, coronary artery disease; CPVT, catecholaminergic polymorphic ventricular tachycardia; CVD, cardiovascular disease; DCM, dilated cardiomyopathy; FH, familial hypercholesterolaemia; HCM, hypertrophic cardiomyopathy; LQTS, long QT syndrome; PRS, polygenic risk score.
♥ Is useful and is recommended.
♥ Can/might be useful and can/might be recommended.
♥ Is not useful and is not recommended. |
Most states in Australia have cardiac genomic clinics who are happy to receive referrals from GPs to not only perform genetic testing, but also to aid in family screening and management. If a P or LP genetic variant is uncovered, specialised genomic clinics can also assist in predictive testing (also known as cascade testing), which would not only identify an at-risk individual who might then benefit from prophylactic management, but also unaffected individuals who can then be discharged from further cardiology follow-up (Figure 1).
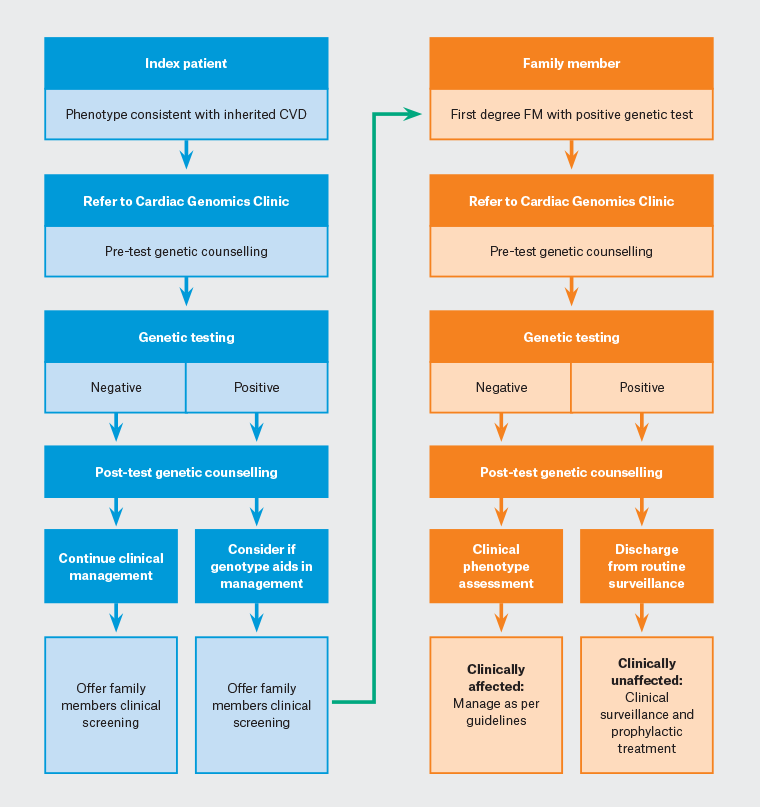
Figure 1. Flow chart for genetic testing in cardiovascular disease. Click here to enlarge
CVD, cardiovascular disease; FM, family member.
With genetic testing now on the MBS, identifying a genomic clinic via one’s local health service would allow patients to be tested with no out-of-pocket expense to them. Meanwhile, there are also multiple resources and registries to assist family members of people with inherited CVDs, including the Australian Genetic Heart Disease Registry, which can provide support to both affected individuals as well as undiagnosed family members.
Implications to consider
Unlike routine laboratory tests, genetic testing results require nuanced interpretation. In this regard, collaboration with genetic counsellors and specialists is vital to ensure accurate assessment and management. Some genetic variants associated with CVDs exhibit variable penetrance, meaning carriers might not necessarily develop the disease until much later in life. This can sometimes lead to lifelong surveillance, but allows the opportunity for lifestyle and environmental modifications to slow the rate of disease progression (eg exercise reduction for ACM). Close liaison with specialists and GPs is therefore required to assist in patient management, remembering that there is a likelihood that multiple members of the same family could be affected.
Genetic testing might unveil unexpected health information and can come with differing levels of psycho-social consequences. For example, predictive testing might reveal non-paternity, which might carry devastating consequences when unexpected. Consequently, appropriate counselling and support for patients grappling with emotionally charged results, while prioritising patient privacy, is required as part of the process. Understanding legal and ethical frameworks surrounding genetic testing, data storage and information sharing is crucial to ensure patient trust and compliance. In Australia, genetic testing is regulated by a combination of federal and state laws, including the Privacy Act 1988, the Therapeutic Goods Act 1989 and state-based health privacy laws.28,29 The Genetic Discrimination Act 2001 is a landmark legislation aimed at preventing genetic discrimination in various areas, including employment and insurance.30 This Act safeguards individuals from being unfairly treated based on their genetic information, assuring patients that their genetic test results will not lead to discrimination.
From a GP’s perspective, all cardiac genomic clinics have genetic counsellors as part of their multidisciplinary team (MDT) approach, which is an invaluable resource when trying to wade through many of these issues. It is anticipated that with the increasing mainstreaming of cardiac genomic clinics, they will become more accessible, with some already offering telehealth services for more regional and remote patients.
Conclusion
Genetics has ushered in a new era of understanding cardiovascular diseases. From a primary healthcare perspective, harnessing genetic knowledge will help improve risk assessment and diagnosis, while holding the promise of tailored treatment strategies in the future. Although current understanding supports only monogenic disease testing at present, which is now accessible via the public healthcare system in Australia, it will be inevitable that genetic information will become more practically useful in the day-to-day health management for our patients. Collaboration between GPs, genetic specialists and genetic counsellors is essential to seamlessly integrate genetics into routine clinical practice. For perhaps very soon, the secrets held within our DNA will find itself centre stage in the battle for global health improvement.
Key points
- Genetics plays an important role in the manifestation of several cardiac diseases, and it is imperative for healthcare professionals to be vigilant in considering referral for genetic evaluation, especially if there is a family history of premature sudden cardiac death (typically in those aged <40 years) or a known inherited cardiac condition.
- Advancements in the understanding of monogenic conditions can provide disease‑specific strategies to prevent disease progression and sudden death, and is currently where genetic testing is most useful.
- Genetic testing for inherited cardiomyopathies, channelopathies and familial hypercholesterolaemia is now available on the Medicare Benefit Scheme (MBS) if requested by a specialist or consultant physician, providing an invaluable tool to aid in screening and management of these possibly life-threatening conditions.
- Cardiac genomic clinics are becoming more available in Australia and can provide support for both clinical management and family screening, as well as providing invaluable multidisciplinary team input, including genetic counselling.
- Currently, polygenic inheritance predictive testing, including risk scores and pharmacogenetics, are not yet robust enough to inform clinical management, but holds great promise for the future.