Injections into the eye are an effective means of treating a number of visual disorders. Although injections can be delivered to a number of different sites, the most common site of injection is into the vitreous cavity at the back of the eye (intravitreal injection [IVI]; Figure 1), and these are an increasingly common way of treating a number of ocular conditions.1,2 Other areas of the eye can also be injected, including the anterior chamber (intracameral injection), but this is less common than IVI therapy.
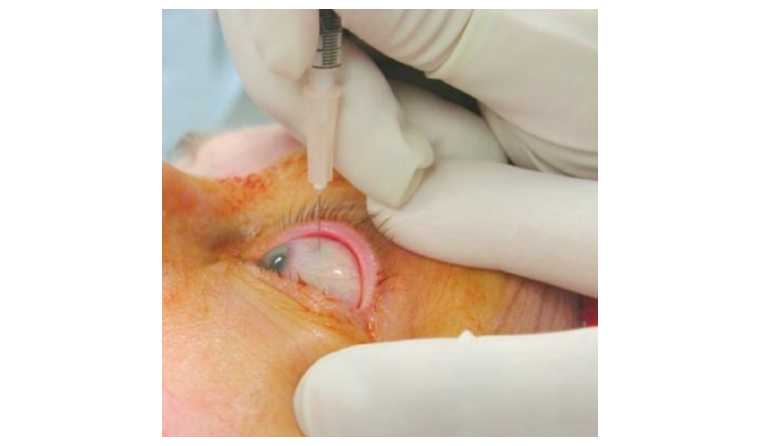
Figure 1. Example of an intravitreal injection.
IVI has become a core component of the treatment of wet (neovascular) age-related macular degeneration (AMD), diabetic macular oedema (DMO) and retinal vein occlusion (RVO), with significant improvements in vision for patients with these conditions. These conditions all involve the formation of oedema within the retina as a result of alterations to retinal vascular function. The medications administered in these conditions are, in general, aimed at decreasing the neovascularisation and vascular leakage that causes this oedema to form, reducing the damage this causes to the retina and improving visual outcomes for those with these diseases. Although safe, there are potential complications that can occur with IVI, some of which might be vision threatening and can even result in loss of the eye.3,4
A range of potential issues can occur after IVI, and when complications do occur, general practitioners are often the first medical professionals patients present to and they might be required to determine which conditions require urgent ophthalmic review and treatment. Here, we focus on the more common complications, such as subconjunctival haemorrhage, postinjection vitreous floaters and corneal abrasion, as well as more serious adverse events, such as an acute rise in intraocular pressure (IOP), vitreous haemorrhage or endophthalmitis.
Subconjunctival haemorrhage
A subconjunctival haemorrhage is a collection of blood between the conjunctiva and the underlying sclera, somewhat similar to a bruise underneath the skin of the hand (Figure 2). Although visually impressive, it is not itself dangerous and will usually resolve spontaneously over a period of one to two weeks.5
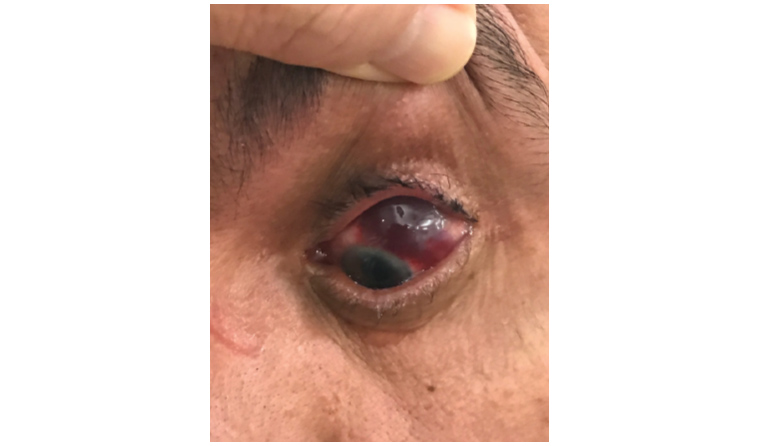
Figure 2. Example of a subconjunctival haemorrhage.
Subconjunctival haemorrhages can happen for a number of reasons, including hypertension and anticoagulant use.3 In some cases they might also be due to a penetrating eye injury and, if this is suspected, urgent referral to an ophthalmologist is recommended. In the context of IVI, subconjunctival haemorrhages are not uncommon and relate to damage to one of the surface blood vessels by the IVI needle. IVI-related subconjunctival haemorrhages are not dangerous and, in the absence of other worrying symptoms, patients can be monitored and reassured that the blood will fade with time. Occasionally, subconjunctival haemorrhages might be associated with some mild irritation, and in these circumstances, the use of lubricating eye drops provides good symptomatic relief.
Vitreous floaters
Many patients notice small bubbles or floaters after injection, sometimes described as appearing ‘like ants crawling across my vision’.6 These are vitreous floaters (similar to those seen in other retinal conditions), and are thought to arise in this case due to either small bubbles within the injected therapeutic and/or regional differences within the medicines that have been injected into the eye.
Floaters that appear immediately after injection are of a different nature to those seen in a vitreous or retinal detachment, and provided that no other worrying symptoms, such as worsening vision or concurrent ocular flashes, are present, these floaters can often be monitored without the need for urgent specialist review. Floaters of this kind generally resolve within 48 hours after injection, and are not associated with other symptoms of retinal detachment, such as curtain-like visual loss.
Corneal/scleral abrasion and irritation
An abrasion of the front of the eye can occur in a number of ways. The IVI needle might scratch a portion of the sclera as it passes into the eye or, alternatively, the ocular surface might become dehydrated while the eye is prepared and sterilised for injection. It is also possible to develop a chemical injury or irritation from the antiseptic used to clean the eye prior to injection, which is usually povidone iodine. Because the ocular surface is highly innervated, damage results in significant pain, usually sharp and made worse by blinking or moving the eye.7,8 Vision may or may not be affected, depending on the site involved.
This condition will usually heal over time and is generally noted by the patient soon after the injection, within a matter of minutes to hours. Treatment with lubricating eye drops or gels can help ease the pain and hasten healing, and the symptoms should improve within a day. If there are any concerns, or any symptoms suggest other possible diagnoses, specialist review should be sought quickly.
Elevated intraocular pressure
Injections into the eye can result in an acute increase in the pressure inside the eye, possibly by chronic damage to the drainage pathways of the eye.9 This is different to the pressure increase seen in most cases of glaucoma, which is gradual in nature. Although the rapid nature of the IOP rise after IVI generally does not harm the optic nerve because it also fades quickly, it can produce severe pain and acutely damage the optical nerve.10,11
The most common presenting symptom for this condition is acute ocular pain in the first few seconds to hours following injection that is not improved by analgesia and that produces a variable degree of visual impairment. Patients with a suspected acute IOP rise should be evaluated by a subspecialist urgently, because IOP lowering needs to be instituted quickly to prevent visual loss.11 In most cases, IOP reduction can be achieved with topical and/or oral therapy, although in some cases, surgical intervention might be necessary to relieve the pressure.12
Vitreous haemorrhage/retinal tear and detachment
Rarely, IVI can be complicated by damage to the periphery of the posterior segment of the eye as the needle passes into the vitreous cavity. If the damage involves a retinal blood vessel, a haemorrhage into the vitreous can result. As blood pools in the normally clear vitreous, it obscures the passage of light to the retina, resulting in significant visual deterioration.
If damage in this area involves the peripheral retina, it can result in a tear of the retina, which, if untreated, can extend to produce a retinal detachment (Figure 3).13 Both vitreous haemorrhage and retinal tear/detachment can exist in isolation or concurrently with each other, and might present similarly. Symptoms include a sudden deterioration in vision, the presence of new floaters and/or ‘flashing lights’, as well as the well-known curtain-like visual loss of retinal detachment.14 Any suspicion of these conditions should suggest the need for urgent referral for ophthalmological review, because prompt surgical treatment often results in better outcomes.
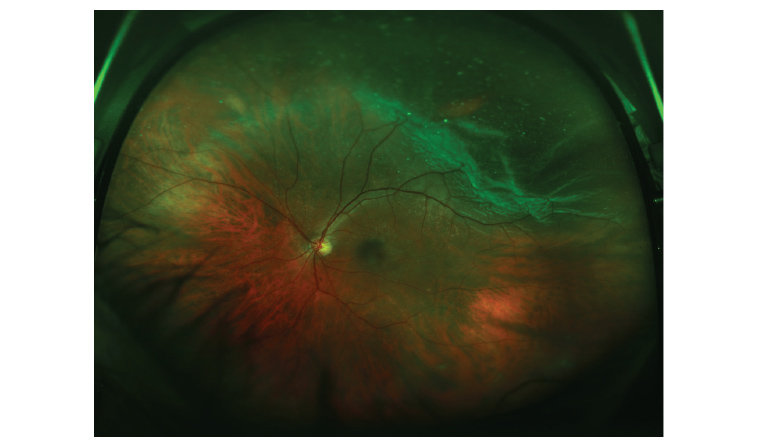
Figure 3. A retinal tear and retinal detachment with vitreous floaters.
Endophthalmitis
Endophthalmitis (Figure 4), or infection inside the eyeball, can occur as a result of IVI, and is thought to relate to the injection itself creating a passage into the eyeball. The species involved in post-IVI endophthalmitis are often highly virulent, and can result in permanent visual loss and, in some cases, blindness.15
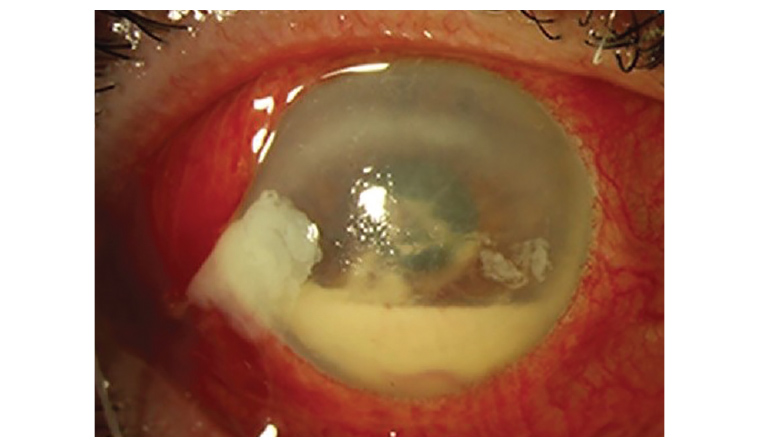
Figure 4. An example of conjunctival injection with a hypopyon in the anterior chamber in a patient with post-intravitreal injection endophthalmitis.
Endophthalmitis usually presents approximately 2–10 days after injection, although it can occur earlier or later than this.16 The initial presenting symptoms are most commonly acute visual deterioration, significant ocular pain and redness of the eyeball.16 Classically, patients will also have a hypopyon, a collection of white blood cells pooling in the anterior chamber of the eye, although this is not always seen, particularly early in the disease process.16,17 Patients might not present with all these signs and symptoms, particularly those who already have significant pre-existing visual impairment, and so any suspicion of endophthalmitis should be urgently referred for specialist evaluation.
Treatment requires urgent intervention by a specialist ophthalmologist, who will conduct intravitreal sampling to identify the organism, as well as intravitreal injection of antibiotics. In many cases, further treatment might be required, with a subspecialist vitreoretinal surgeon needing to undertake surgical removal of the infected vitreous via a vitrectomy operation.18
Conclusion
Injections into the eye represent an expanding area of ophthalmic treatment and are generally well tolerated. General practitioners are often the first point of call for patients should problems arise. The presence of worrying symptoms, particularly severe pain, changes in vision and flashing and/or floaters might indicate the presence of serious complications, and should prompt urgent referral for ophthalmic review.
Key points
- Intravitreal injection is one of the most common outpatient procedures in Australia.
- Serious complications are rare but can occur, and include infection and retinal tear.
- Warning signs of potential serious problems include severe pain and vision change.
- If these occur, urgent ophthalmic review should be sought.