Obstructive sleep apnoea (OSA) is a condition characterised by upper airway obstruction during sleep, resulting in repetitive complete or partial breathing pauses (apnoeas or hypopnoeas, respectively), oxygen desaturations and arousals from sleep. The presence and severity of OSA is usually characterised using the apnoea–hypopnoea index (AHI), representing the average number of apnoeas and hypopnoeas per hour of sleep.
The prevalence of OSA (AHI ≥5/hour) in the general population is high,1 especially in older adults. Moderate–severe OSA, defined as an AHI >15/hour, affects 20% of middle-aged men and 10% of middle-aged women.2 Obstructive sleep apnoea/hypopnoea syndrome (OSAS), defined as OSA plus symptoms of excessive daytime sleepiness, affects 5–8% of the middle-aged population.2 The prevalence of OSA is increased in patients with type 2 diabetes, hypertension and cardiovascular disease (CVD). OSA costs the Australian community $18.9 billion annually, including from direct health system costs, secondary health conditions and productivity losses.3
Common risk factors for OSA are outlined in Box 1.4
| Box 1. Risk factors for OSA |
- Gender: male
- Age: over 50 years
- Overweight, obesity and metabolic conditions, including glucose intolerance, insulin resistance and type 2 diabetes
- Excess alcohol consumption
- Sedative medication
- Opioids
- Smoking
- Sleeping in supine position
- Tonsillar hypertrophy
- Nasal obstruction
- Craniofacial abnormalities (eg retrognathia)
- Thyroid disease
- Neuromuscular disease
- Insomnia
- Family history, including genetic factors related to jaw morphology
- Postmenopause (women)
|
Reproduced from Australasian Sleep Association. Presentation and risk factors. Australasian Sleep Association, 2024. Available at www.sleepprimarycareresources.org.au/osa/presentation-and-risk-factors [Accessed 22 March 2024], with permission from the Australasian Sleep Association.4
OSA, obstructive sleep apnoea. |
Given the high prevalence of OSA and its association with medical comorbidities (eg obesity, hypertension and diabetes), a large number of patients presenting to general practitioners (GPs) will have OSA symptoms and/or risk factors. It is important to determine which patients warrant further assessment and the need for sleep testing. Many patients might be asymptomatic, minimally symptomatic, or have only mild disease and be at low risk of adverse sequelae. These patients might not benefit from medical treatment apart from management of their risk factors (eg treatment of obesity and reduction in alcohol consumption). However, some patients with mild OSA will have troublesome daytime symptoms and might benefit from a trial of therapy, such as continuous positive airway pressure (CPAP).
Untreated OSA can have major adverse effects on health and wellbeing and is associated with negative health outcomes such as increased risks of CVD, cognitive decline, motor vehicle accidents (MVAs) and depression. These patients will likely benefit most from treatment. For instance, patients with resistant hypertension often have OSA as a contributing factor so should be appropriately investigated and managed for OSA in addition to the usual hypertension interventions.
Furthermore, 30–40% of patients with insomnia meet the diagnostic criteria for OSA and approximately 30–50% of OSA patients report clinically significant insomnia symptoms. Comorbid OSA and insomnia (COMISA) results in greater morbidity, including daytime impairments and depressive symptoms, compared to patients with either condition alone.5,6 Sweetman et al address the management of COMISA.7 OSA can also overlap with other sleep disorders, including restless legs syndrome and periodic limb movement disorder, and require additional therapy.5,6
Box 2 outlines the roles that GPs have in diagnosing and managing OSA.8 Historically, GPs referred patients mainly to hospital-based clinics and some private specialists. Increasing recognition and demand for services led to commercialisation of testing. However, access to diagnosis and treatment can be difficult.9 There have also been changes to Medicare Benefits Schedule (MBS) rules enabling GPs to directly order a sleep study without prior review by a respiratory/sleep specialist.
| Box 2. Roles of the general practitioner in the diagnosis and management of OSA |
- Assess patients for the presence of OSA and, if necessary, refer patients to specialists or directly order sleep studies (depending on complexity and severity and availability of services)
- Manage snoring and offer lifestyle advice, including sleep health, diet and physical activity
- Manage hypertension, cardiovascular system risk factors and other comorbidities
- Monitor adherence to treatment and direct patients to a local distributor for equipment issues regarding CPAP
- Prescribe other treatment if required, with referral to relevant specialists (eg dentists, ENT specialists)
|
Reproduced from Australasian Sleep Association. Obstructive Sleep Apnoea: Introduction. Australasian Sleep Association, 2024. Available at www.sleepprimarycareresources.org.au/osa/introduction [Accessed 22 March 2024], with permission from the Australasian Sleep Association.8
CPAP, continuous positive airway pressure; ENT, ear, nose, throat; OSA, obstructive sleep apnoea. |
The remainder of this article aims to describe the diagnostic pathways for OSA in general practice, including OSA screening tools and referral pathways for sleep studies in relation to current MBS funding criteria.
Aim
The aim of this paper is to improve awareness of common risk factors for and clinical presentation of OSA in primary care to improve patient health outcomes. The paper seeks to understand how screening tools, such as the OSA50 questionnaire and the Epworth Sleepiness Scale, can help GPs identify patients who are at high risk for OSA with significant daytime sleepiness.
Diagnostic pathway
Symptoms, differential diagnoses and clinical presentation
Patients with OSA might themselves be unaware that they have a sleep disorder and often present due to concerns from their bed partner or family members regarding loud snoring, witnessed apnoeas and restless sleep, or they might be worried about potential adverse health consequences. Patients might report episodes of choking arousals from sleep, disrupted sleep with frequent nocturnal awakenings or nocturia. Potential daytime symptoms include waking unrefreshed, excessive sleepiness and frequent naps or dozing off easily, poor concentration, mood disturbance and fatigue. Some patients might present after an MVA related to excessive sleepiness. Men will often present with classically described symptoms of snoring, apnoeas and excessive daytime sleepiness, whereas women might present with less specific symptoms of fatigue, depression and insomnia.10
Daytime symptoms of OSA are, however, shared with other conditions. Chronic sleep restriction, an insufficient time spent in bed to allow for sleep due to family, social or work demands, is an important cause of daytime sleepiness. Sleepiness and fatigue might also be due to other conditions, including anaemia, diabetes mellitus, hypothyroidism, mood disorders or medication side effects. Other sleep disorders associated with increased daytime somnolence include narcolepsy and idiopathic hypersomnolence, and a delayed sleep phase might present with difficulties waking up and sleepiness during the morning hours. Further evaluation and consultation with a sleep specialist might need to be considered, particularly if testing does not show significant OSA or there is no response to a trial of therapy.
Physical examination should include assessment of body mass index (BMI), waist circumference (WC) and neck circumference (increased risk of OSA is associated with BMI ≥30 kg/m2; WC male ≥102 cm, female ≥88 cm; neck circumference male ≥42 cm, female ≥39 cm); blood pressure; cardiorespiratory examination; inspection of the nose and throat for reduced nasal patency (eg nasal polyps or rhinitis) or a crowded pharyngeal airway (tonsillar enlargement or macroglossia); or craniofacial risk factors (eg retrognathia, micrognathia, maxillary hypoplasia or acromegalic facies). The Sleep Health Primary Care Resources website, an ‘accepted clinical resource’ of the Royal Australian College of General Practitioners, illustrates the modified Mallampati score.11 A high Mallampati score of III or IV represents a predisposing factor for OSA, especially if associated with nasal obstruction.
OSA screening questionnaires and the Epworth Sleepiness Scale
OSA screening questionnaires, such as the OSA50,12 STOP-Bang13 and Berlin questionnaires,14 can help GPs to identify patients who are at high risk of having OSA. The OSA screening questionnaires have similar diagnostic accuracy for detection of moderate–severe OSA and are highly sensitive but have low specificity (ie high false positive rate) for detecting moderate–severe OSA (Table 1). Thus, a positive screening questionnaire result by itself does not confirm a diagnosis of OSA, and the patient should proceed to sleep study testing. Both the OSA50 and Berlin questionnaires were developed and validated for use in primary care; however, the OSA50 is often favoured due to its brevity and simple scoring system. Furthermore, unlike the Berlin and STOP-Bang questionnaires, the OSA50 was specifically developed using an Australian primary care population. Although also simple to use, the STOP-Bang was originally designed for OSA screening in a preoperative clinic setting rather than primary care; however, similar predictive performance in general practice has been demonstrated.17
| Table 1. Diagnostic accuracy of screening tools for identification of moderate–severe OSA |
| |
Sensitivity |
Specificity |
| OSA50 ≥512 |
94 |
31 |
| STOP-Bang ≥315 |
90 |
36 |
| Berlin questionnaire (high risk)16 |
82 |
39 |
| Epworth Sleepiness Scale >1015 |
47 |
62 |
| OSA, obstructive sleep apnoea. |
OSA50 questionnaire
The OSA50 questionnaire is brief and easy to use, consisting of only four items (Figure 1). An OSA50 score ≥5 out of 10 indicates a high risk of moderate–severe OSA.

Figure 1. OSA50 screening questionnaire.
Reproduced from Chai-Coetzer CL, Antic NA, Rowland LS, et al. A simplified model of screening questionnaire and home monitoring for obstructive sleep apnoea in primary care. Thorax 2011;66(3):213–19, with permission from BMJ Publishing Group.12
STOP-Bang questionnaire
The STOP-Bang questionnaire consists of eight questions with a yes/no response, based on the following symptoms and risk factors for OSA:13
- Snoring
- Tiredness
- Observed apnoeas
- High blood Pressure
- BMI >35 kg/m2
- Age >50 years
- Neck circumference >40 cm
- Male Gender.
The presence of at least three items is indicative of a high risk for OSA.
Berlin questionnaire
The Berlin questionnaire was designed specifically for use in primary care and consists of 11 items within three categories:14
- snoring and witnessed apnoeas (category 1)
- daytime sleepiness or fatigue (category 2)
- self-reported hypertension and obesity (category 3).
Responses are used to categorise patients as either having a high risk or low risk for OSA.
Epworth Sleepiness Scale
The Epworth Sleepiness Scale (ESS) provides a subjective rating of the degree of daytime sleepiness experienced by a patient.18 It asks a patient to rate their chances of dozing off (using a score of 0–3) in eight commonly encountered scenarios, resulting in a total score out of 24 points. Higher scores indicate greater degrees of sleepiness. An ESS score ≥8, used in MBS criteria for sleep study item numbers (see the section below on sleep study testing), is generally indicative of at least mild daytime sleepiness. The ESS score has not been shown to be a reliable predictor of an OSA diagnosis, as many patients with moderate–severe OSA do not experience excessive sleepiness. However, it is useful in identifying patients who are symptomatic from their OSA and likely to benefit from a trial of therapy. The ESS is also useful for monitoring a patient’s response to treatment, especially if they present with a complaint of daytime sleepiness.
Sleep study testing
Sleep studies are generally divided into one of four ‘levels’ of testing, depending on the number of recording channels and whether the study is attended by a sleep scientist (laboratory based) versus in an unattended setting (eg in home).19 It is important to note that the quality of sleep studies can be highly variable between testing sites. Sleep laboratories that have gained accreditation by the National Association of Testing Authorities (NATA) meet minimal standards for sleep disorders services that have been set by the ASA.
Levels of sleep study testing
Level 1: Laboratory-based, full polysomnography (PSG) with ≥7 recording channels that includes measurement of sleep – the gold standard
Level 2: Unattended, full PSG with ≥7 recording channels, usually conducted in the patient’s own home
Level 3: Limited cardiorespiratory recording (4–6 channels), without measurement of sleep
Level 4: Limited recording with only 1–3 channels (including oximetry), without measurement of sleep
Medicare rebates are currently available for level 1 or 2 full PSG but not levels 3 or 4 limited-channel sleep studies. If GPs wish to directly request a PSG, the patient needs to have a high risk of symptomatic, moderate–severe OSA, as indicated by a positive response to an OSA screening questionnaire (an OSA50 ≥5, STOP-Bang ≥3 or ‘high risk’ on the Berlin questionnaire) plus at least mild daytime sleepiness (ESS ≥8). Otherwise, the patient will need to be referred for assessment by a respiratory/sleep physician prior to a sleep study being conducted. A flowchart outlining the clinical pathways for a patient with suspected OSA in relation to current Medicare criteria is presented in Figure 2.
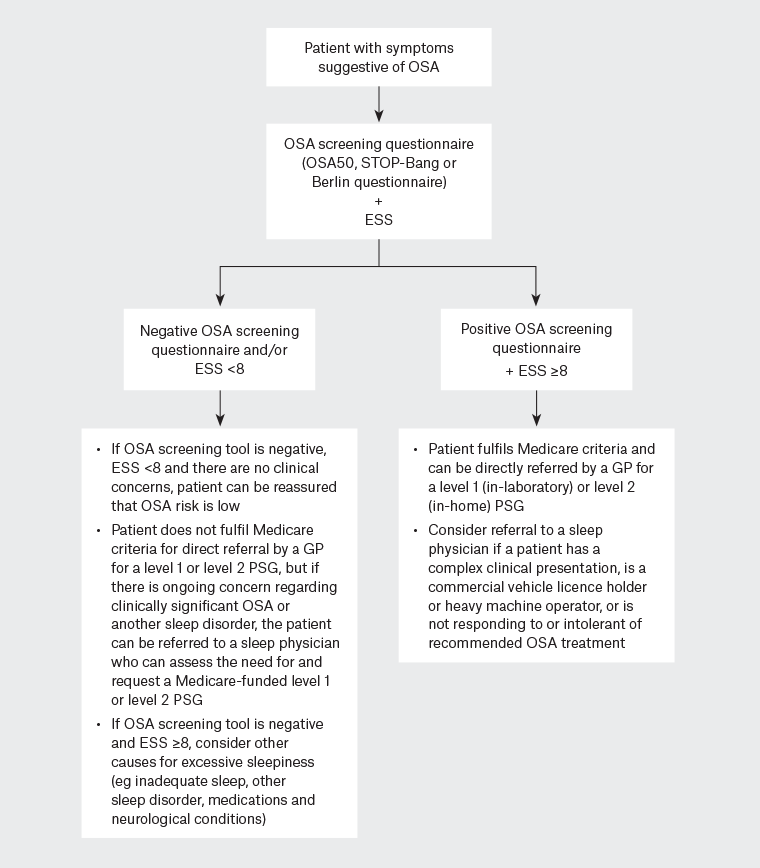
Figure 2. Flow chart of diagnostic pathways for OSA in primary care.
ESS, Epworth Sleepiness Scale; GP, general practitioner; OSA, obstructive sleep apnoea; PSG, polysomnogram.
Referral to a sleep physician
Referral to a sleep physician should be considered if there is a high clinical suspicion for OSA despite a negative response to an OSA screening questionnaire and/or an ESS score <8 or if there are symptoms suggestive of another disorder affecting sleep (eg insomnia, parasomnia, narcolepsy or periodic limb movement disorder). A specialist opinion should also be sought if the patient has a complex presentation, including ESS ≥16 (ie severe daytime sleepiness) that does not improve with standard OSA therapies or when the underlying cause is unclear; sleepiness-related motor vehicle or work accident or high occupational risk (eg commercial drivers, pilots, heavy machinery operators or those holding a heavy vehicle licence [gross vehicle mass >4.5 tonnes]); BMI ≥45kg/m2; alcohol abuse; chronic opioid use; neuromuscular disease; significant respiratory disease (eg severe COPD); or heart failure.20 If a patient is noncompliant with or unable to tolerate recommended treatment or remains symptomatic despite effective therapy for OSA, further evaluation by a sleep physician is recommended.
Conclusion
General practitioners play an important role in the diagnosis and management of patients with OSA, and awareness of the risk factors and presenting symptoms of this highly prevalent sleep disorder can improve patient health and wellbeing. Patients at high risk of symptomatic, moderate–severe OSA, identified using screening tools such as the OSA50 questionnaire, STOP-Bang and/or ESS, can be directly referred by GPs for an in-laboratory or in-home PSG. Patients with a high clinical suspicion for OSA or other sleep disorders but who do not meet MBS criteria for direct referral for sleep study testing, those with complex presentations or significant medical comorbidities, and heavy vehicle licence holders can be referred to a specialist sleep physician for further assessment. Management of OSA in primary care is discussed in a subsequent article by Ellender et al in this issue of the Australian Journal of General Practice.21
Key points
- OSA is a highly prevalent sleep disorder with important adverse health consequences.
- Awareness of the common risk factors and clinical presentation of OSA in primary care settings can improve patient health outcomes.
- Screening tools (eg OSA50, STOP-Bang and ESS) can help identify patients at high risk of symptomatic moderate–severe OSA.
- Patients who meet MBS criteria might be directly referred by GPs for sleep study testing.
- Sleep physician referral should be considered for patients with complex presentations or other suspected sleep disorders as well as heavy vehicle licence holders.