Within this issue of the Australian Journal of General Practice, there is a separate article overviewing the epidemiology, clinical presentation and investigation options available to establish a diagnosis of obstructive sleep apnoea (OSA).1 This companion article focuses on the overall principles to consider when reviewing a patient in general practice following a confirmed diagnosis of OSA. OSA management options are outlined, and the important area of driving safety for patients with OSA is reviewed.
Aim
This article examines the management options for a patient with an established diagnosis of OSA to provide a guide for driving licensing requirements. Indications for continuous positive airway pressure (CPAP) are discussed and tips are provided to consider when conducting a review appointment, including trouble shooting.
Overall principals of OSA management
OSA is a common disorder that has all the characteristics of a chronic condition.2 There are many disease management issues for patients with OSA, including lifestyle factors known to contribute to OSA severity; multiple comorbidities; low CPAP therapy adherence; and cost considerations.2 Mental health issues such as depression are also associated with sleep apnoea. Poor health literacy is potentially a major barrier to optimal disease control.3 According to the adult OSA Task Force of the American Academy of Sleep Medicine clinical guidelines for 2009, OSA should be approached as a chronic disease requiring long-term, multidisciplinary management.4 Integrated care for OSA involves collaboration between health professionals from a variety of disciplines, including general practitioners, nurses and sleep specialists. Strategies aimed at promoting patient self-management and improving communication between patients and healthcare providers, including structured, chronic disease self-management programs and use of e-health interventions and related technologies, might benefit patients with OSA.5
OSA treatment options
The major treatment options available for symptomatic OSA are positive airway pressure therapy, mandibular advancement splints, sleep apnoea surgery, positional therapy and weight loss. Most patients with symptomatic moderate-to-severe OSA will require treatment with CPAP therapy. However, the selection of optimal therapy is based on interpreting the diagnostic sleep study results (to define the severity of respiratory events and the degree of nocturnal oxygen desaturation), the clinical history (eg degree of sleepiness, motor vehicle crash risk and presence of comorbid sleep diagnoses, including insomnia) and the presence of relevant medical comorbidities (eg cardiovascular and respiratory diseases). This approach is outlined pictorially in Figure 1. The severity of OSA is currently defined from the sleep study using the apnoea–hypopnoea index (AHI), which represents the number of complete or partial breathing pauses per hour of sleep accompanied by either oxygen desaturation or cortical arousal. A comprehensive description of OSA treatment options and supporting evidence can be found on the Primary Care Sleep Health Resource website (www.sleepprimarycareresources.org.au).
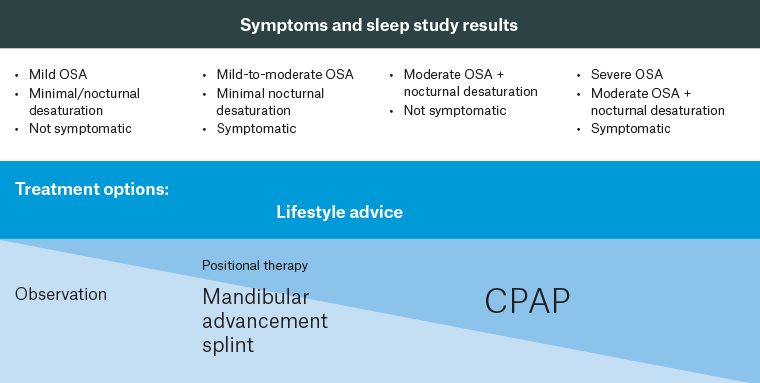
Figure 1. Approach to first-line OSA treatment.
A patient’s symptoms and sleep study results (top) can be used to guide management approaches for OSA (bottom).
CPAP, continuous positive airway pressure; OSA, obstructive sleep apnoea.
Lifestyle advice
Weight loss significantly and independently reduces both AHI and excessive daytime sleepiness (EDS),6 and a 10% weight loss can be expected to cause a clinically meaningful reduction in AHI.7 Furthermore, weight loss has established benefits with respect to cardiovascular risk reduction and should be actively promoted for all overweight and obese patients with OSA. Long-term follow-up data out to four years suggests that for every kilogram of weight loss long term, there is a sustained reduction in AHI (0.78 events/hour for every kilogram of weight lost).8,9 There is insufficient evidence at this time to recommend glucagon-like peptide 1 receptor (GLP-1) agonists as an isolated therapy.10 However, this is an evolving potential future therapeutic approach. The results of a randomised control trial with tirzepatide (combination GLP-1 agonist and glucose-dependent insulinotropic polypeptide agonist) are expected to be published mid-2024, which might result in changes to this recommendation.11 Dietary and exercise interventions have further benefits, including improved sleep quality and arousal threshold independent of body mass index (BMI) reduction.12 Alcohol reduction also improves OSA severity in addition to providing other benefits for BMI and sleep quality.13
Bariatric surgery in adults with moderate-to-severe OSA and BMI >35kg/m2 improves the oxygen desaturation index and EDS (mean improvement in Epworth Sleepiness Scale [ESS] was −5), with a mean reduction in AHI of 23 events/hour compared to conservative care.14
CPAP therapy
CPAP is a small bedside pump that uses positive pressure to splint open the upper airway and prevent collapse. The machine is connected to the face via a hose and a mask. The masks can be nasal, nasal pillow, full face (also called oronasal) or over-the-mouth hybrid in design. Positive pressure can be delivered through four main types/algorithms: continuous (CPAP), auto-titrating (auto-titrating positive airway pressure [APAP]), adaptive servo-ventilation (ASV) or bilevel positive airway pressure (BiPAP). APAP is a form of CPAP in which the device contains sensors to increase and decrease the pressure to maintain a patent airway. BiPAP machines are ventilators that alternate between inspiratory and expiratory pressures; these devices are used for hypoventilation disorders or, rarely, for OSA where very high pressures are required. ASV devices are ventilators with proprietary algorithms that track breathing throughout the inspiratory and expiratory cycle to achieve a targeted tidal volume; these machines are used for central sleep apnoea in sleep specialist clinics and are not recommended for patients with low left ventricular ejection fractions.
Although APAP is generally required to initiate positive pressure therapy at home to determine ongoing CPAP pressure requirements, most OSA patients do not require APAP devices long term; these machines tend to be more expensive and there is no additional benefit for these devices long term over fixed pressure CPAP.8 BiPAP is reserved for sleep and medical disorders associated with hypoventilation or selected OSA patients requiring very high pressures.
Indications
CPAP is the first-line treatment for most adults with symptomatic moderate-to-severe OSA or mild OSA with significant hypoxemia and/or EDS.15 There is high-grade evidence that CPAP improves AHI and moderate-to-high-grade evidence for improvements in ESS and quality of life.15 Other potential benefits include improved anxiety and depression symptoms, motor vehicle crash risk, atrial fibrillation recurrence and blood pressure control.15 Randomised control trial data has not shown, thus far, any cardiovascular secondary prevention benefits (cardiovascular events or mortality) associated with CPAP treatment in adults with moderate-to-severe OSA and minimal sleepiness.16
OSA treatment benefits are dose dependent, with four hours per night CPAP usage representing the lower limit for effective therapy across populations, although some individuals will benefit at use levels lower than this.17 Table 1 outlines important topics to cover at a CPAP review appointment. The goal of optimal therapy is to achieve an ESS <10 and improvement in EDS symptoms such as reduced need for naps, waking refreshed. Driving safety is crucial to review and is discussed below. Table 2 describes the approach to driver’s licence assessment for patients with confirmed OSA.
| Table 1. CPAP review appointment checklist at 2, 6 and 12 months after commencing therapy |
| |
Ideal response |
Action if ideal response not met |
| Symptoms |
- Sleep refreshing
- Less/no need for afternoon nap
- ESS <10
|
Check download for usage >4 h/night and check for side effects and other factors that affect adherence. If side effects and mask fit are optimised and the patient is still not achieving ideal response, recommend referral for sleep specialist review |
| Driving safety |
- No inattention with driving
- No micro sleeps
- No sleepiness/fatigue related accidents or near-miss accidents
|
Sleep specialist review |
| CPAP download |
- >4 h usage per night, 70% of nights
- Machine-derived AHI <5/h
- Mask leak <20–30L/min (depending on machine brand)
|
Check for side effects, mask seal and pressure tolerance. Consider referral back to CPAP provider for mask review. Consider comorbid insomnia symptoms that can contribute to reduced CPAP adherence. If side effects and mask fit are optimised and the patient is still not achieving ideal response, consider sleep specialist referral |
| Side effects |
- Minimal aerophagia, pressure tolerance concerns, dry mouth or dry eyes.
|
Check mask fit and cleaning routines. CPAP provider review for mask fit. If optimised and still not achieving ideal response, recommend referral for sleep specialist review |
| AHI, apnoea–hypopnoea index; CPAP, continuous positive airway pressure; ESS, excessive daytime sleepiness. |
Side effects and other factors that affect adherence
The major barrier to long-term OSA outcomes is CPAP adherence, with a large proportion of patients struggling to maintain usage for more than four hours per night at 12 months.18 Patients particularly at risk of non-adherence are those with socioeconomic disadvantage and low educational backgrounds, comorbid insomnia symptoms and mental health diagnoses and those with low symptom burden.19 Common side effects include dry eyes, dry mouth, claustrophobia, nasal drip and noisy mask. Optimising mask fit is the cornerstone of therapy usage, and often side effects can be remedied by improving mask cleaning to improve the seal and mask fit techniques. Many of the units have proprietary settings that can be utilised to improve comfort, including the addition of heated humidification or changing the length of time the CPAP machine ramps (ie the machine uses a lower pressure at treatment onset and slowly increases to the target pressure over a variable time period). There are several evidence-based interventions that can be used to improve CPAP use, including education, motivational interviewing, cognitive behavioural therapy, peer-to-peer support and group format interventions.15
Approximately 30–50% of patients with OSA report clinically significant insomnia symptoms.20 Among OSA patients with comorbid insomnia symptoms, initial treatment of insomnia with cognitive behavioural therapy for insomnia improves insomnia symptoms and might facilitate improved acceptance and long-term use of CPAP therapy.20
Costs
CPAP funding varies around Australia and New Zealand by region and state. Many states will have government-funded CPAP programs through local public hospitals for concession card or Health Care Card holders. Most of these programs will require eligible patients to rent CPAP for a period to confirm CPAP adherence, with often a requirement to purchase a mask – this initial cost might vary between $100 and $500.
The cost of self-funding a CPAP machine varies among retailers, from $900 to $2000. Many private health funds will rebate between $500 and $1000 for set-up costs. Masks generally require replacement every three years, with silicon mask cushions and head straps requiring replacement approximately every 12 months depending on cleaning routines.
Telemedicine and CPAP
CPAP modems have the capacity to transfer and store information to cloud-based servers, enabling health providers to remotely access an individual’s CPAP data, identify poor CPAP adherence and intervene when needed. Furthermore, data can be transferred directly to patients via specialised online portals to provide feedback on CPAP use to enhance self-management, such as seeking additional support from clinicians or CPAP providers. Although such technologies have significant potential to improve the care of patients with OSA, monitoring of patient data can be labour intensive, and only limited evidence exists to support their role in patient management.21,22
Mandibular advancement splints
Mandibular advancement splints (MAS), also known as mandibular advancement devices (MADs), are oral appliances worn during sleep to protrude the lower jaw and prevent airway collapse. MAS should be custom made by a dentist qualified and experienced in dental sleep medicine and who preferably holds a Fellowship of Dental Sleep Medicine postgraduate qualification. Non-customised ‘boil and bite’ devices are usually not as effective, nor tolerated as well as custom devices.23 MAS therapy is associated with improvements in AHI (moderate grade evidence), ESS (low grade evidence) and oxygen saturation. Symptomatic improvements might occur even without normalisation of AHI.23
Indication
For adults with adequate dentition, good oral health and mild or moderate OSA without significant oxygen desaturation, MAS can be considered as the first-line therapy.24 For patients with other severities of OSA who are intolerant of CPAP, MAS should be considered over no therapy.24
Side effects
Most side effects related to custom-fitted devices are temporary and include excess salivation, dry mouth, discomfort around the teeth, gums or temporomandibular joint pain, or irritation to the soft tissues of the mouth, particularly in the first fortnight of use. In the long term, MAS can be associated with bite change, so regular review and monitoring by a dentist is a necessary component of ongoing MAS therapy.
Australian OSA treatment costs
There is no Australian Medicare funding for the purchase of MAS/MADs. There can be access for concession card or Health Care Card holders through public dental programs or public hospital dental units in some states. However, most programs include some co-payment for the device, usually between $400 and $900. Most patients will have to see a private dentist and self-fund this therapy, with costs varying from $1200 to $2100. The device will usually last four or more years depending on the manufacturer, type and cleaning routine. Many private health insurance companies will rebate between $300 and $1000 from ancillary type extras cover for the overall therapy.
Positional therapy
Positional therapy can be used for OSA where upper airway obstruction is present predominantly in the supine position. Various approaches can be taken to prevent supine sleep, including the application of vibro-tactile electronic devices, sewing tennis balls into a nightshirt and using pillows with straps. However, there is insufficient data available to compare electronic and mechanical devices. These devices can be considered for adults with mild-to-moderate OSA, without significant hypoxia and where there are infrequent events in the lateral position (eg lateral AHI <15/hour). They are also most likely to be acceptable to patients when snoring is limited to the supine position. Although there are few side effects, the major barrier is low long-term adherence. The evidence for these devices is currently low or very low grade.25 Costs vary from $150 to $400 depending on the type of device.
Upper airway surgery
Upper airway surgery such as uvulopalatopharyngoplasty and maxillomandibular advancement surgery (MMA) remains largely a last resort for adults with OSA when patients cannot tolerate first-line treatments.4 Prior to this treatment, other reversible factors should be addressed (eg weight loss). However, case selection is essential and results are best for patients with mild disease, presence of craniofacial abnormality (for MMA), low BMI or notable upper airway crowding (eg large tonsils). Sleep specialist input is strongly encouraged prior to referral for surgery outside these indications as the decision requires balancing surgical risks and the limited longevity of surgical efficacy over time as people age or gain weight.4 Hypoglossal nerve stimulators are implanted devices that activate the hypoglossal nerve synchronised with a patient’s breathing to keep the airway patent during sleep. These devices are on the horizon in Australia; however, a sleep specialist and experienced sleep surgeon should have a multidisciplinary approach when considering these devices.
Novel targets for therapy based on pathophysiological endotype
A major issue in managing patients with OSA is the substantial inter-individual variability in treatment responses to various modalities, including CPAP. There is currently active research into understanding OSA physiological endotypes that contribute to upper airway collapse and how knowledge of these endotypes could guide selection of current and novel therapies. Although this new approach is currently a research tool, it might enable individualised approaches to optimise treatment success in the future.
Significant research efforts are currently under way to test new targeted therapies for OSA, including drug therapies (eg reboxetine) targeting low arousal threshold.26–29 However, there is insufficient evidence to support replacing current standard therapies with these new therapies for OSA.
Following sleep specialist consultation, wake promoting agents might be indicated in combination with first-line therapy (CPAP or MAS) where excessive daytime somnolence persists despite adequate usage.30 Agents that are currently approved (but not funded for this indication) in Australia are modafinil or armodafinil and internationally are solriamfetol and pitolisant (not yet registered by the Therapeutic Goods Administration).
Assessing OSA and driving risk in primary care
OSA is associated with impaired driving performance31,32 and at least a two-fold increase in workplace33 and motor vehicle accident risk,34 likely driven by EDS. Although it is clear that OSA patients as a group have EDS, there is growing evidence of substantial inter-individual variation.35–37 Because of this heterogeneity, assessing fitness to drive or work with OSA is challenging, further complicated by the fact that practitioners rely on patients honestly reporting any symptoms of impairment. As such, a comprehensive history of sleep, daytime function and driving history should be undertaken prior to any discussion about the consequences of sleep-related impairment. Clinical daytime sleepiness testing, including the multiple sleep latency test (MSLT) and particularly the maintenance of wakefulness test (MWT), are used in sleep specialist settings to objectively evaluate a patient’s tendency to fall asleep or ability or stay awake. However, these tests are not suitable for use in primary care due to the complexity and cost of such tests and are isolated to use in specialist sleep services.
From the perspective of primary care management of OSA-related driving risk, it is important to have appropriate triaging of at-risk patients. The approach to triaging would depend on whether a patient with OSA holds a private versus a commercial driver’s licence; a more conservative approach and sleep specialist review are required for commercial licence holders. For patients with a private driver’s licence, a positive screen on the OSA50 or STOP-Bang questionnaire and severe excessive daytime sleepiness (ESS ≥16) should trigger a high risk for driving with the need for expedited assessment and treatment. Referral to a sleep specialist should be considered if there is uncertainty regarding best management or there are driving-related concerns. For example, asking the patient about motor vehicle accidents or near-miss accidents, particularly relating to fatigue in the past 12 months, would further strengthen the suspicion of high driving risk and need for sleep specialist referral.
For patients with a commercial driver’s licence, the approach should be more cautious and conservative. This is because there is significant underreporting of sleepiness symptoms in this population.38–41 Therefore, relying on subjective sleepiness measures is problematic and a referral to sleep specialist assessment is required for licensing. Table 2 describes the assessment pathways for private and commercial licence holders. For more detailed information, see the 2022 edition of the guidelines on assessing fitness to drive.42 Investigations and OSA treatment can be immediately commenced in primary care, which can allow a commercial driver to continue duties while awaiting sleep specialist input, providing the treating doctor does not feel there is a significant driving risk as a result of the sleep disorder.
| Table 2. Approach to driver’s licence assessment for patients with confirmed OSA |
| Private driver’s licence holders |
Action |
Commercial driver’s licence holders |
Action |
- Confirmed OSA and ESS ≥16; or presence of high-risk features in the historyA
|
- Perform sleep study, implement treatment and refer to sleep specialist
- Implement driving restriction until confirmed adequate treatment response, or urgent sleep specialist review
- Only eligible for conditional licence once on therapy
|
- OSA50 ≥5 or ‘yes’ response to 3–8
- STOP-Bang questions; or
- Confirmed OSA
|
- Perform sleep study, implement treatment and refer to sleep specialist
- Give general driving advice as per Austroads guidelines
- Interim driving restrictions if high-risk features are present in the historyA
|
- Confirmed OSA
- ESS <10
- No high-risk features in historyA
|
- Implement appropriate treatment as clinically indicated
- Eligible for unconditional licence
|
|
|
AHigh-risk features42 that would warrant recommending a driving restriction include a history of accidents or near-miss accidents relating to sleepiness; or frequent self-reported episodes of sleepiness or drowsiness while driving; or if the person, in the opinion of the treating doctor, represents a significant driving risk as a result of a sleep disorder.
ESS, Epworth Sleepiness Scale; OSA, obstructive sleep apnoea. |
Screening questionnaires can be found on the Primary Care Sleep Health Resource website (www.sleepprimarycareresources.org.au/osa/questionnaires).
Indications for sleep specialist referral
As discussed in the companion article in this issue by Chai-Coetzer and Hancock,1 if a patient does not fulfill the ESS and screening questionnaire requirements to access a Medicare-billed diagnostic sleep study, a specialist review is required for a sleep study referral. Other indications for specialist review are:
- commercial drivers
- presence of sleepiness-related accidents or near-miss episodes
- BMI >45 kg/m2
- alcohol abuse
- neuromuscular disease
- significant respiratory disease (severe chronic obstructive pulmonary disease)
- significant cardiovascular disease (heart failure).
Conclusion
Treatment of OSA aims to improve symptoms (sleepiness, quality of life, mood and disruptive snoring), blood pressure control and disease severity and reduce motor vehicle crash risk. Matching the patient with the optimal first-line therapy requires an assessment of disease severity and symptoms. Achieving optimal treatment adherence is essential for improved outcomes, and a chronic disease model of multidisciplinary support can assist in achieving long-term treatment success. Sleep specialist referral is required in complex cases and where treatment response is suboptimal.