Genitourinary symptoms are reported by up to 75% of breast cancer survivors.1 Symptoms include vaginal dryness, itching, burning, pain and irritation; dysuria; urinary urgency, nocturia, incontinence; recurrent urinary tract infections; and dyspareunia. The aetiology is chronic oestrogen depletion, often worse in women with breast cancer due to endocrine therapies and treatment-induced early menopause. Oestrogen depletion causes changes to the vulvovaginal and bladder–urethral tissues, with a resulting decrease in blood flow and secretions, increased pH, thinning of the epithelium and loss of elasticity.2 Unlike other menopausal symptoms, genitourinary symptoms are chronic and progressive, and can be a significant concern for breast cancer survivors. Vaginal oestrogen therapy can assist with the management of genitourinary symptoms, but women and their clinicians can find decision making challenging.
Most breast cancers are hormone receptor (oestrogen and/or progesterone) positive, for which oestrogen blockade and deprivation form the mainstay of treatment. Women with early-stage hormone receptor-positive breast cancers are typically prescribed 5–10 years of endocrine therapy after surgery, with or without radiotherapy, and those with higher-risk cancers might also receive chemotherapy.3
The endocrine therapies used in early breast cancer are tamoxifen and aromatase inhibitors (anastrozole, letrozole and exemestane).3,4 Their different modes of action are shown in Figure 1. Tamoxifen is a selective oestrogen receptor modulator that blocks oestrogen uptake at the oestrogen receptor.4 As such, it is effective in both pre- and postmenopausal women. Aromatase inhibitors reduce peripheral oestrogen production by inhibiting the aromatase enzyme.4 Their efficacy depends on near-total suppression of oestrogen.4 Aromatase inhibitors are effective in postmenopausal women where the ovaries are no longer functioning, and in premenopausal women with ovarian function suppression via monthly gonadotropin-releasing hormone (GnRH) agonist or bilateral oophorectomy.3,4
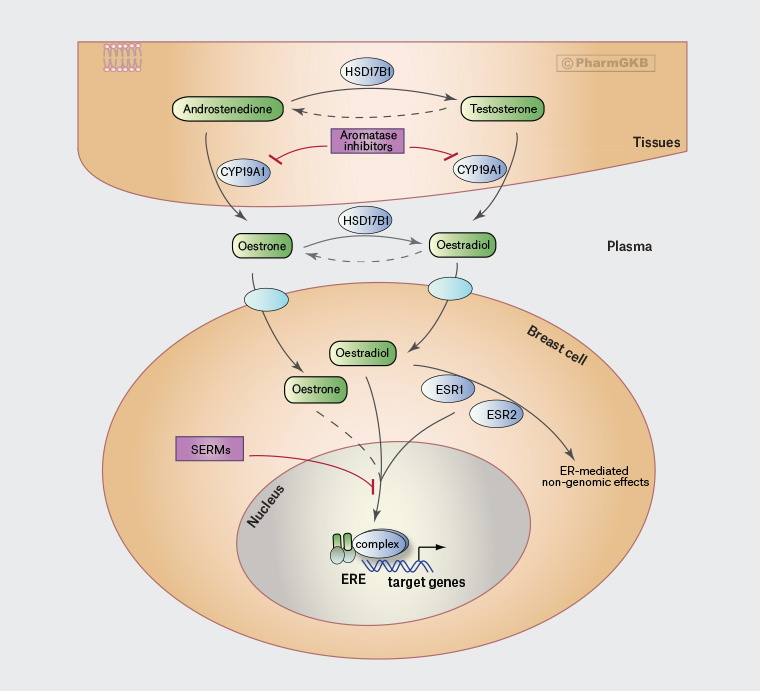
Figure 1. Location of action of aromatose inhibitors and selective oestrogen receptor modulators. Click here to enlarge
Adapted from Whirl-Carrillo M, McDonagh EM, Hebert JM, et al. Pharmacogenomics knowledge for personalized medicine. Clin Pharm Therap 2012;92(4), with permission from PharmGKB.
CYP19A1, cytochrome P450 family 19 subfamily A member 1; ER, oestrogen receptor; ERE, oestrogen response element; ESR1, oestrogen receptor 1; ESR2, oestrogen receptor 2; HSD17B1, 17β-hydroxysteroid dehydrogenase 1; SERMs, selective oestrogen receptor modulators. Empty ovals on top of the breast cell are transporter genes.
In postmenopausal women, aromatase inhibitors are more effective than tamoxifen in reducing the risk of breast cancer recurrence and death.5 Among premenopausal women, adding ovarian function suppression to tamoxifen or an aromatase inhibitor improves disease-free and overall survival compared with tamoxifen alone.6
Although hormone receptor-positive breast cancer survival outcomes are better with the lower oestrogen levels achieved from aromatase inhibitors and/or ovarian suppression, lower oestrogen levels are associated with more menopausal side effects, including genitourinary symptoms, and these side effects might impair quality of life and reduce adherence to endocrine therapy.7
Vaginal administration of oestrogen is very effective for preventing and treating urogenital atrophy;8 however, the potential for systemic oestrogen absorption and stimulation of dormant micrometastases means the safety of use in women with breast cancer is debated. Because of this, initial treatment with non-hormonal vaginal lubricants and moisturisers is recommended.8,9 In fact, in one study in postmenopausal women with no history of breast cancer and moderate-to-severe genitourinary symptoms, the regular use of vaginal moisturiser (Replens; Church and Dwight [Australia] Pty Ltd) was just as effective as oestradiol 10 mcg tablets in reducing the most bothersome symptoms.10
Types of vaginal oestrogens
Table 1 summarises vaginal oestrogen formulations available in Australia. Patients are often aware of options not available in Australia and might source a private supply for personal use. Other medications that patients might be aware of are dehydroepiandrosterone (DHEA) and ospemifene. DHEA (also known as prasterone) vaginal pessaries are approved by the Australian Therapeutics Good Administration (TGA), but not yet available in Australia. Ospemifene, taken orally, is a partial oestrogen agonist (selective oestrogen receptor modulator), but is not yet TGA approved. For both products, there is evidence of efficacy, but the safety in breast cancer survivors has not yet been established.11,12
| Table 1. Types and doses of vaginal oestrogens available in Australia |
| Oestrogen |
Trade name and formulation |
Dose |
| Oestradiol |
Vagifem® Low pessary (Novo Nordisk Pharmaceuticals Pty Ltd) |
10 mcg/dose |
| Oestriol |
Ovestin cream, 1 mg/g (0.1%)
Ovestin Ovula pessary (Aspen Pharmacare
Australia Pty Ltd) |
500 mcg/dose
500 mcg/dose |
Oestriol or oestradiol
The vaginal oestrogen products available in Australia contain either oestradiol or oestriol, and both can be used in women with breast cancer. Oestriol is a less potent oestrogen and cannot be converted to oestradiol.13 The metabolic clearance of oestriol is more rapid14 and systemic absorption lower.15 These differences give oestriol a theoretical advantage over oestradiol for women with hormone receptor-positive breast cancer, for whom systemic oestrogen concentrations should be kept very low. However, there are no clinical outcome data to support superior safety or efficacy of one type of vaginal oestrogen over another in women with breast cancer. Oestriol can still act as a systemic oestrogen if serum concentrations are consistently elevated.16
Systemic absorption from vaginal oestrogens
The absorption of vaginal oestrogen varies depending on mucosal health and thickness, the efficacy of normal vaginal barriers such as mucus, contact time, dose frequency and location (oestrogens deposited higher using an applicator are absorbed more than those deposited nearer the introitus).17,18 When vaginal oestrogens are first prescribed, systemic absorption typically rises initially and then falls rapidly as the vaginal epithelium thickens, which generally occurs over two to four weeks, but can take longer for women on aromatase inhibitors.19 It can be seen from Figure 1 that any systemically absorbed oestrogen should be blocked at the site of action by tamoxifen, whereas it bypasses aromatase conversion and therefore continues to act in women on aromatase inhibitors. Switching from an aromatase inhibitor to tamoxifen should therefore be discussed as a risk-mitigation measure for women wishing to commence vaginal oestrogens. The switch can also improve genitourinary symptoms because tamoxifen causes less severe vaginal dryness.20 However, switching to tamoxifen must be balanced against the superior breast cancer recurrence and survival advantages provided by aromatase inhibitors.5,6
The dose of oestrogen in most Australian vaginal oestrogen preparations is very low. For example, 10 mcg oestradiol pessaries (Vagifem Low; Novo Nordisk Pharmaceuticals Pty Ltd) deliver a typical serum concentration of 4.6 pg/mL (the normal postmenopausal oestradiol reference range is <10 pg/mL).21 To put this into perspective, the total systemic dose of oestrogen per year when 10 mcg oestradiol pessaries are used twice weekly is comparable to a single 1-mg oestradiol oral tablet.21
Measuring oestrogen concentrations
There is no evidence to support measuring serum oestrogen concentrations in routine practice to assess systemic absorption of vaginal oestrogens. For women on aromatase inhibitors, there is no established minimum level of circulating oestradiol that has been linked to an increased risk of breast cancer recurrence.9
Safety of vaginal oestrogens in women with breast cancer
To prove vaginal oestrogens are safe in women with early-stage breast cancer, a large prospective randomised control trial with long-term follow-up to assess breast cancer recurrences and deaths is required. No such trials have been, or likely ever will be, conducted.
A meta-analysis on the safety of vaginal oestrogens in women on aromatase inhibitors for early breast cancer included 11 studies (two randomised, nine observational) with sample size ranging from seven to 61 participants.22 Only a small number of premenopausal women on ovarian function suppression with aromatase inhibitors were included. Vaginal preparations containing both oestradiol (five studies) and oestriol (three studies) were included. The authors concluded that vaginal oestrogens had no significant effect on oestradiol levels compared with baseline, but none of the included studies reported breast cancer relapse and mortality rates.22
A case-control study from the UK looked at the safety of vaginal oestrogens in women on endocrine therapy for early-stage breast cancer.23 The authors found no increased risk of breast cancer recurrence in 259 women on tamoxifen using vaginal oestrogens (7% had a recurrence) compared with 8478 women on tamoxifen without vaginal oestrogens (9% had a recurrence).23 Unfortunately, there were insufficient women on aromatase inhibitors to determine the impact of vaginal oestrogens in these women.
A recent Danish observational cohort study reported on 8461 women with early breast cancer diagnosed between 1997 and 2004.24 The majority (63%) were taking adjuvant endocrine therapy, 1957 (23%) were prescribed vaginal oestrogens (oestradiol pessary, oestradiol vaginal ring, oestriol pessary, oestriol cream) and 133 (2%) were prescribed menopausal hormone therapy with or without vaginal oestrogens.24 With a median of 9.8 years of follow-up, there was no increased risk of breast cancer recurrence or mortality with vaginal oestrogens or menopausal hormone therapy.24 However, a subgroup analysis revealed an increased risk of recurrence, but not mortality, in women receiving vaginal oestrogens with adjuvant aromatase inhibitors (adjusted relative risk of recurrence 1.39; 95% confidence interval: 1.04–1.85).24 This was not seen in the women on tamoxifen. The limitations of that study are that it was not randomised, the number of women on aromatase inhibitors was small and these women might have been at higher risk of recurrence compared with those chosen for tamoxifen, and there are no details on the type, dose and duration of use of the vaginal oestrogens. Given oestradiol 10 mcg tablets replaced the 25 mcg tablets in 2010, many of the women in this study will have been on higher doses of vaginal oestrogens than are typically used today.
Another cohort study from the UK with 49,237 females with breast cancer and a median duration of follow up of eight years reported no evidence of higher breast cancer-specific mortality in those who used vaginal oestrogen therapy after breast cancer diagnosis (n=2551, 5%) compared with those who did not, including women taking aromatase inhibitors.25 Similarly, a Swedish case-control study showed no increase in breast cancer-specific mortality in patients with breast cancer who used oestrogen concurrent with tamoxifen and aromatase inhibitors.26 Although reassuring, neither study reported on breast cancer recurrences and the follow-up times were short to report on early breast cancer mortality.
In summary, the existing evidence is reassuring for women on tamoxifen or no adjuvant endocrine therapy who are considering using vaginal oestrogen. The situation for women using aromatase inhibitors is less certain and further studies are needed to assess safety.
A patient-centred approach to decision making
Women with breast cancer who are bothered by genitourinary symptoms face a difficult decision when considering vaginal oestrogen therapy. General practitioners can assist by making an assessment including a thorough history and offering an examination, ensuring that non-hormonal measures are in place, helping to quantify the individual woman’s risk of breast cancer recurrence and discussing the known evidence around the use of vaginal oestrogens. This enables a shared decision-making process where the woman can make a decision that takes into account the severity of her symptoms, her cancer type and risk of cancer recurrence, the type of endocrine therapy she is using (if any) and her personal preferences and values. For women on aromatase inhibitors, including premenopausal women on GnRH agonists plus aromatase inhibitors, switching to tamoxifen (with or without stopping the GnRH agonist) can help.
For women whose symptoms do not respond to non-hormonal remedies, low-dose vaginal oestrogens can be considered in the form of oestradiol pessaries, oestriol pessaries or oestriol cream. The type of oestrogen and preparation will depend on individual patient and clinician preferences. Some women find the tablets easier to use, whereas others prefer the cream because it can be targeted to areas of most concern. Insertion into the lower third of the vagina is preferable to maximise response and minimise absorption.17,18 Treatment is usually commenced with daily use for 14 days, then twice weekly use for long-term maintenance. Our approach to vaginal oestrogen use in women with different types of breast cancer and treatments is summarised in Table 2. General practitioners treating women on aromatase inhibitors are advised to discuss proposed vaginal oestrogen therapy with the patient and her oncology team.
| Table 2. Summary of prescribing recommendations for vaginal oestrogens3,8,9,27 |
| Type of breast cancerA |
Vaginal oestrogen use |
| Hormone receptor negative |
|
| Hormone receptor-positive breast cancer in women currently taking tamoxifen |
|
| Hormone receptor-positive breast cancer in premenopausal women currently taking GnRH agonist with tamoxifen |
- Can be prescribed
- (Stopping the GnRH agonist might also improve symptoms and can be considered after discussion with the woman and her oncology team)
|
| Hormone receptor-positive breast cancer in women currently taking aromatase inhibitors |
- Oncologist consultation recommended
- Consider switch to tamoxifen
- If tamoxifen contraindicated, not tolerated or not preferred, consider vaginal oestrogens after a discussion of the potential risks and benefits with each individual woman, in consultation with her oncology team. Start with a 12-week trial and consider longer-term use if significant improvement in symptoms
|
| Hormone receptor-positive breast cancer in premenopausal women currently taking GnRH agonist with aromatase inhibitor |
- Oncologist consultation recommended
- Consider switch to tamoxifen+GnRH and, if no improvement, stop the GnRH agonist and continue tamoxifen alone (after discussing the potential risks and benefits with the woman and her oncology team)
- If tamoxifen contraindicated, not tolerated or not preferred, consider vaginal oestrogens after a discussion of the potential risks and benefits with each individual woman, in consultation with her oncology team. Start with a 12-week trial and consider longer-term use if significant improvement in symptoms
|
| Hormone receptor-positive breast cancer in women who have completed or stopped adjuvant endocrine therapy (usually 5–10 years after diagnosis ) |
|
ARecommendations are similar for women with early- and advanced-stage breast cancers.
GnRH, gonadotropin-releasing hormone. |
Conclusion
The initial treatment of women with genitourinary symptoms and a history of breast cancer should be non-hormonal vaginal moisturisers and lubricants. If vaginal oestrogen is being considered, an individualised discussion is required with each woman, balancing the risk of cancer recurrence with the efficacy of non-hormonal therapies, severity of genitourinary symptoms and associated quality of life.
Key points
- Genitourinary symptoms are common in women with breast cancer.
- Initial management should be with non-hormonal lubricants and moisturisers.
- Vaginal oestrogens can be offered to women with persistent symptoms.
- An individualised discussion of the potential risks and benefits is required.
- Safety is uncertain in women with early breast cancer on aromatase inhibitors.