Intraductal papillary mucinous neoplasms (IPMNs) of the pancreas are premalignant cystic epithelial tumours of the pancreatic duct and/or its side branches. These tumours produce mucin, resulting in ductal dilatation.1 The prevalence of IPMN and other pancreatic cystic lesions (PCL) has increased due to better quality imaging and its increasing usage.2 PCL are identified in up to 75% of the general population and become more prevalent with increasing age.3,4 The true incidence of IPMN is not known, because the majority of patients are asymptomatic. However, IPMNs account for 0.5% of malignant pancreatic neoplasms found at autopsy, 7.5% of clinically diagnosed pancreatic neoplasms and 16–25% of surgically resected pancreatic neoplasms.5 There are no data on the incidence of PCL or IPMN in Australia. The typical age of IPMN diagnosis is between the fifth and seventh decades, with a slight preponderance for males.6 IPMN might be multifocal in up to 40% of cases.7
Classification and natural history
IPMN is divided morphologically into three subtypes: main duct (MD-IPMN), branch duct (BD-IPMN) and mixed type (MT-IPMN). MD-IPMN is associated with segmental or diffuse dilatation of the main pancreatic duct to ≥5 mm in size, without other obstructive causes (Figure 1). BD-IPMN involves cystic dilatations ≥5 mm of a side branch duct, with visible communication to the main pancreatic duct (Figure 2). MT-IPMN involves both the main pancreatic duct and its side branches, and has a risk profile similar to MD-IPMN.9 There are four histological subtypes with varying malignant potential, but these are currently indistinguishable radiologically.6

Figure 1. Main duct intraductal papillary mucinous neoplasm in the body and tail of the pancreas.
Reproduced from Morana G, Ciet P, Venturini S. Cystic pancreatic lesions: MR imaging findings and management. Insights Imaging 2021;12:115, with permission from Springer.8
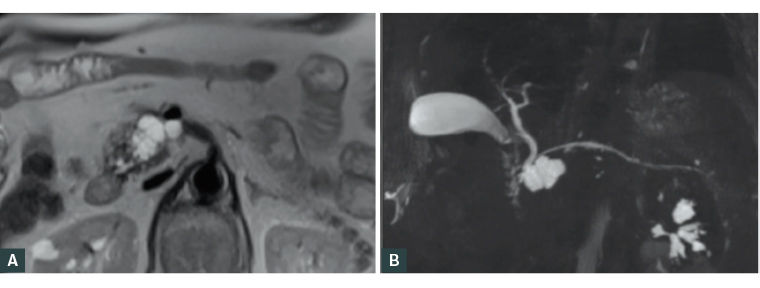 Figure 2. Branch duct intraductal papillary mucinous neoplasm in the head of the pancreas.
Figure 2. Branch duct intraductal papillary mucinous neoplasm in the head of the pancreas.
(A) T2-weighted magnetic resonance imaging image of the cystic lesion in the head of the pancreas. (B) Magnetic resonance cholangiopancreatography image of the cystic lesion in the head of the pancreas.
Reproduced from Morana G, Ciet P, Venturini S. Cystic pancreatic lesions: MR imaging findings and management. Insights Imaging 2021;12:115, with permission from Springer.8
Invasive carcinoma can arise within IPMN through the adenoma–carcinoma sequence from low- to high-grade dysplasia, then to invasive carcinoma.2 Historical data on IPMN malignant risk is difficult to interpret due to changes over time in imaging quality and usage. Older surgical studies report a wide malignancy rate of 6–51% in BD-IPMN and 35–100% in MD-IPMN.10 However, recent observational studies suggest that malignancy risk might be lower, leading to a more conservative approach to resection.11–13 A recent systematic analysis found that approximately 20% of patients with low-risk BD-IPMN progressed to worrisome features or high-risk stigmata during their surveillance.11 Of those with progression, just over half (11.8% of the entire cohort) underwent surgery, and 29.5% of those who underwent surgery had malignancy present on final pathology.11 This equated to a pancreatic malignancy rate of 2.7%/year.11 These malignancies were not all associated with the IPMN, because 20% of cases were concomitant pancreatic duct adenocarcinoma (PDAC) located separate to IPMN (Figure 3).11 Because not all patients with high-risk stigmata undergo surgery, the overall malignancy rate for IPMN might be different to that seen in resected specimens.
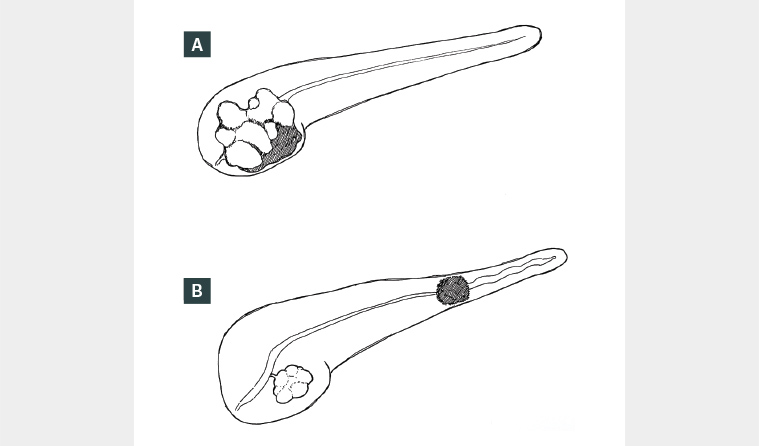
Figure 3. (A) Intraductal papillary mucinous neoplasm (IPMN)-associated malignancy. (B) IPMN (uncinate) with concomitant pancreatic ductal adenocarcinoma (tail).
Invasive IPMN is associated with a better five-year overall survival (43–60%)14 compared with ordinary PDAC (~8% in Australia).15 The reason for this difference is not clear, but is likely multifactorial. Through surveillance, invasive IPMN is likely diagnosed at an earlier stage, although longer overall survival has been reported in early stage (N0M0) invasive IPMN compared with PDAC (43 vs 12.8 months, respectively).6,16,17 Invasive IPMN is associated with more indolent colloid carcinoma subtype and less aggressive histopathological features (eg lower rates of positive nodes and perineural invasion).16,17
Risk factors for malignant transformation
A family history of PDAC increases the risk of developing malignancy in patients with IPMN. The presence of one first-degree relative (FDR) with PDAC increases the risk 2.3-fold; two FDRs increases the risk 6.4-fold and three or more FDRs increases the risk 32-fold.9 Known familial conditions associated with IPMN and pancreatic cancer include BRCA2 mutations and Fanconi, familial atypical mole malignant melanoma and Peutz–Jeghers syndromes.9 Other potential risk factors include obesity, smoking (≥20 pack-years) and long-standing diabetes.18–20 The standardised incidence ratio (compared with the general population) of PDAC for IPMN is 5, whereas that for IPMN in the presence of diabetes is 12.20 Patients with IPMN have an increased risk of concomitant PDAC (4–10%), and this should be considered during surveillance.20,21 Outside known cancer syndromes, there is no association between the presence of IPMN and the risk of development of other extrapancreatic cancers.22
Presentation
Most IPMNs are asymptomatic, with the vast majority detected incidentally on imaging performed for unrelated indications. Up to one-third of patients report vague abdominal symptoms, although it is not usually possible to attribute these symptoms to IPMN. Symptoms might develop when complications occur, such as acute pancreatitis from mucinous plugging.2 Invasive IPMN might present like PDAC, with back and/or abdominal pain, jaundice, weight loss and new-onset diabetes.2,10
Diagnosis
Once IPMN is suspected on imaging, it must be differentiated from other PCLs and the risk of malignancy stratified. Other PCLs include pseudocysts, mucinous cystic neoplasms and serous cystadenoma.14 Differentiating IPMNs from other PCLs can be difficult and often relies on a combination of history (eg pancreatitis) and imaging characteristics. High-quality imaging of the pancreas with either magnetic resonance imaging (MRI) or a computed tomography (CT) scan of the pancreas is vital. MRI with contrast is preferred initially for its superiority in assessing mural features (thickening, nodules), internal architecture (septations, solid components) and ductal features (dilatation, strictures, communication).22 The questions governing investigations for potential IPMN are: (1) is the cyst currently malignant; (2) is it a mucinous cyst; and (3) what is its risk of malignant transformation? A management algorithm for suspected IPMN is shown in Figure 4.
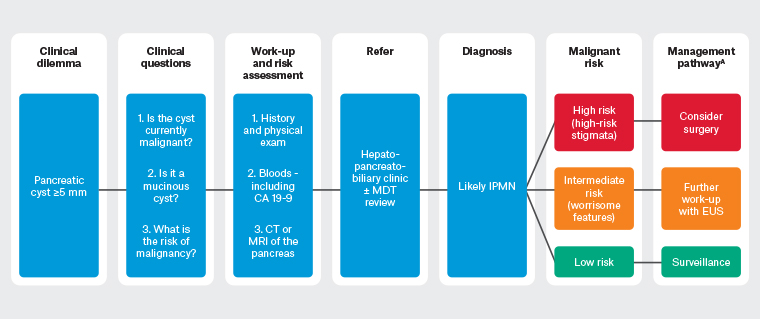
Figure 4. Management algorithm for suspected intraductal papillary mucinous neoplasm of the pancreas. Click here to enlarge
A Consider no further intervention/surveillance in patients who are poor surgical candidates.
CA19-9, carbohydrate antigen 19-9; CT, computed tomography; EUS, endoscopic ultrasound;
IPMN, intraductal papillary mucinous neoplasm; MDT, multidisciplinary team; MRI, magnetic resonance imaging.
The goal of IPMN management is to detect potentially preventable or curable invasive carcinoma.23 There are numerous guidelines on the management of IPMN based on consensus opinions due to a lack of high-level evidence.10 As such, there are significant variations between the guidelines. The International Association of Pancreatology (Fukuoka) guidelines9 and European guidelines22 are commonly used in Australia. These feature clinical, biochemical and radiological criteria to risk stratify IPMNs as low risk or those with worrisome features or high-risk stigmata (Table 1).24 The presence of high-risk stigmata and individual worrisome features have a positive predictive value for malignancy of 56–89% and 27–33%, respectively.22 Risk stratification guides further management (Figure 4).
| Table 1. High-risk stigmata and worrisome features of intraductal papillary mucinous neoplasm of the pancreas based on Fukuoka9 and European22 guidelines |
| High-risk stigmata (consider resection) |
Clinical
|
Imaging
- Enhancing mural nodule ≥5 mm
- Main pancreatic duct ≥10 mm
|
| Positive cytology for malignancy |
| Worrisome features (evaluate further with EUS) |
Clinical
- Pancreatitis
- New-onset diabetesA
|
Imaging
- Cyst ≥3 cm or ≥4 cmA
- Enhancing mural nodule <5 mm
- Thickened or enhancing cyst wall
- Main pancreatic duct 5–9 mm
- Abrupt change in calibre of pancreatic duct with distal pancreatic atrophy
- Regional lymphadenopathy
- Cyst growth rate ≥5 mm/2 years or 5 mm/yearA
|
| Biochemical
|
ARisk factor in European guideline only.
CA19-9, carbohydrate antigen 19-9; EUS, endoscopic ultrasound. |
Although these guidelines have high sensitivity for malignancy, their specificity is low. For example, the Fukuoka guidelines have an overall sensitivity of 73% and specificity of 46% for predicting advanced neoplasia.24 It is estimated that approximately 75% of resections for IPMN will be for non-invasive lesions, which could otherwise be monitored.3 Currently, achieving a balance between over- and under-treatment remains difficult.
Further investigations
Endoscopic ultrasound
Endoscopic ultrasound (EUS) is used to evaluate indeterminate cysts and IPMNs with worrisome features.9 EUS can confirm the diagnosis of IPMN, assess for high-risk features and gain cyst fluid for analysis.
Cyst fluid analysis
Standard cyst fluid analysis includes cytology, microscopy for the presence of mucin and measurement of amylase, carcinoembryonic antigen (CEA) and glucose levels. Cytology might identify malignant cells (specificity ~94%, sensitivity 51%).25 The presence of mucin is specific for a mucinous cyst. Elevated cyst fluid CEA (>192 ng/mL) is diagnostic of a mucinous lesion, but does not predict invasive disease.26 Fluid DNA analysis for KRAS and GNAS mutations might be more accurate for diagnosing IPMN;27 however, its role is not well defined in the diagnosis or risk stratification of IPMN.10
Serum tumour markers
There is no role for serum CEA or carbohydrate antigen (CA) 19-9 in the diagnosis of IPMN; however, they might help differentiate benign from invasive IPMN. Serum CA19-9 >37 U/ml has a sensitivity of 74% and specificity of 86% for invasive disease. Serum CEA >5 mcg/L has a sensitivity of 40% and specificity of 92%.9
Positron emission tomography/CT
Positron emission tomography/CT is not used for the diagnosis of IPMN. Its use in risk stratification is not well defined.10
Endoscopic retrograde cholangiopancreatography and pancreatoscopy
Endoscopic retrograde cholangiopancreatography is not part of standard IPMN work-up. It is typically reserved for relief of obstructive jaundice, which might be caused by malignant transformation. Pancreatoscopy might aid in assessment of the extent of disease, but is not widely available and not routinely used.28
What is the management of IPMN?
IPMNs are managed conjointly by pancreatic surgeons and gastroenterologists and discussed in a multidisciplinary meeting if there are worrisome or high-risk features.
Surveillance
Low-risk lesions should undergo surveillance. Surveillance imaging protocols vary between guidelines. Most protocols in Australia and New Zealand are based on the Fukuoka9 or European22 guidelines (Table 2). Poor surgical candidates do not need ongoing surveillance, because multimorbid patients are 11-fold more likely to die from other causes.29
| Table 2. Recommended surveillance strategy for intraductal papillary mucinous neoplasm of the pancreas according to European22 and International Association of Pancreatology/Fukuoka9 guidelines |
| |
European guidelines |
Fukuoka guidelines |
| Recommended imaging modality |
MRI |
CT scan or MRI |
| Branch duct cyst size (cm) |
|
|
| <1 |
6-monthly for 1 year, then yearly until no longer a surgical candidate |
6-monthly for 1 year, then every 2 years |
| 1–2 |
6-monthly for 1 year, then yearly for 2 years and then every 2 years |
| 2–3 |
EUS in 3–6 months, then alternate with MRI yearly |
| >3 |
Alternate EUS and MRI every 3–6 months |
| CT, computed tomography; EUS, endoscopic ultrasound; MRI, magnetic resonance imaging. |
Surgery
Due to the increased risk of malignancy in MD-IPMN, MT-IPMN and BD-IPMN with high-risk stigmata, surgery is offered to those fit for resection.9,22 High-risk features include cysts with confirmed malignancy or likely to harbour malignancy, such as in the presence of enhancing mural nodules >5 mm or dilated pancreatic duct >10 mm.9,22 Depending on the site of the IPMN, the surgery offered might be pancreaticoduodenectomy, distal pancreatectomy or total pancreatectomy. Total pancreatectomy might be considered for MD-IPMN because it often involves the entire pancreatic duct. There is no evidence for neoadjuvant chemotherapy for IPMN, even with malignant transformation.22
Surveillance following resection
Ongoing surveillance of the remanent pancreas is recommended due to the risk of developing further IPMN or malignancy.9,22 The risk of IPMN recurrence is approximately 7–20%.22
Screening
There is currently no role for screening for IPMN, even in the presence of known risk factors.
Conclusion
IPMN is a premalignant condition with increasing prevalence, although the majority of IPMNs carry a low likelihood of malignancy. Risk stratification is predominantly done with imaging. Management should be individualised, with resection considered for high-risk individuals and surveillance for low-risk individuals.
Key points
- IPMN is increasing in prevalence, likely due to the increased frequency and quality of cross-sectional imaging.
- IPMN is a premalignant condition (transformation risk ~2%/year), but its true malignant potential is uncertain.
- Invasive IPMN has a better prognosis than ordinary pancreatic ductal adenocarcinoma.
- Assessing the risk of malignancy of IPMN relies on surveillance imaging over time.
- Patient-centred management is vital in achieving a balance between over- and under-treatment.