Despite being a preventable and curable disease, tuberculosis (TB) is one of the world’s deadliest diseases. Worldwide, TB is the 13th leading cause of death and the second leading infectious killer after COVID-19 (above human immunodeficiency virus [HIV] and acquired immunodeficiency syndrome [AIDS]).1 In 2021, TB claimed 1.6 million lives and 10.4 million people were diagnosed with the disease. Current TB control efforts are not adequate, with COVID-19 causing a serious setback. Innovative approaches are needed for its control and prevention.
TB is a bacterial infection caused by Mycobacterium tuberculosis that might lead to active TB disease or total healing or, most often, a persistent state of TB infection (TBI). Active TB disease can cause severe morbidity with long-term sequelae. In the absence of treatment, it often leads to death from complications, especially in resource-limited high burden countries (HBCs). In contrast, TBI is neither symptomatic nor infectious, with approximately 5–10% of those with TBI progressing to active TB disease. In low burden countries (LBC), where TB transmission is sporadic rather than endemic, progression from past TBI, often acquired overseas, together with localised outbreaks around those cases, is the dominant source of new cases of active TB disease. TBI screening and treatment can play a significant role in the prevention of active TB disease, thereby avoiding serious and potentially fatal consequences, as well as preventing secondary cases through transmission.
One of the top 10 priority indicators for the End TB Strategy of the World Health Organization (WHO) is TBI treatment coverage of equal to or more than 90%.2
The two types of tests used to detect TBI are the tuberculin sensitivity test (TST) and interferon gamma release assay (IGRA). Either TST or IGRA is recommended to test for TBI in high-income and upper middle-income countries with estimated TB incidence of fewer than 100 per 100,000.3
Australia has maintained good TB control since the 1980s, with an average of five to six cases of TB disease per 100,000 population. There were 1438 TB notifications in Australia in 2018.4 This has remained almost static for more than three decades. In Australia, as in other LBCs, almost 90% of active TB cases occur from progression of remote past TBI.4 With increased global movement through travel and migration and the growing numbers of international students, refugees and asylum seekers moving from HBCs to Australia, TB control and prevention will pose further challenges.
Current policies and programs for prevention and treatment of TB in Australia include Bacillus Calmette–Guérin (BCG) vaccination, border screening, management of immune compromised conditions and contact tracing. With the above approaches, the annual case numbers in Australia are expected to fall to 38 new cases per million population by 2050,5 far behind the WHO’s elimination target of one or less than one new case per million population per year by 2050.2
Screening is a common approach to TB control,6 and, along with treatment of TBI, might help to achieve the WHO’s elimination target. It provides an opportunity to detect TBI before it progresses to active TB, and on treatment completion, the likelihood of progression to active TB disease is markedly reduced.7 As primary care is the first point of contact with the healthcare system for most people, there is the opportunity for TBI screening and treatment to be delivered and managed in primary care settings in LBCs, including Australia.5,8–10
Typically, the steps (cascade of care) involved in the implementation of screening and treatment of TBI are the identification of the at-risk population, application of a screening test, TBI diagnosis, treatment initiation, follow-up and treatment completion. See Figure 1 for an illustrated version of the cascade of care. A simplified flowchart for key steps for general practice management of TBI has also been created by Denholm et al.5
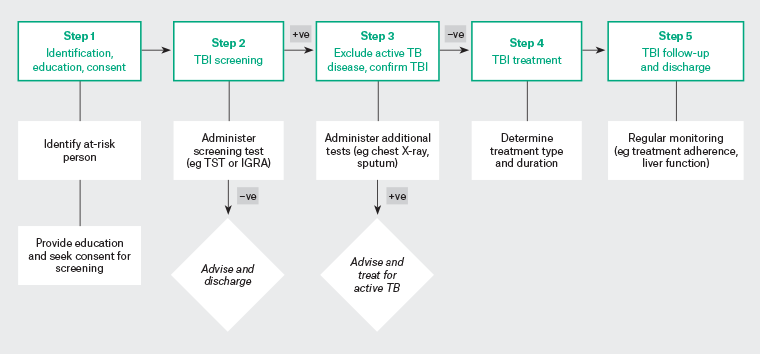
Figure 1. Main steps of the cascade of care for TBI screening and treatment. Click here to enlarge
IGRA, interferon gamma release assays; TB, tuberculosis; TBI, tuberculosis infection; TST, tuberculin sensitivity test; +ve, positive; −ve, negative.
Although there is strong global recommendation in this direction, there is no consensus on how TBI screening and treatment could be undertaken in primary care. Although systematic reviews have been published on aspects of TBI, such as global prevalence,11 cascade of care,12 health effects13 and treatment,14 research is limited on models of care in the setting of general practice in LBCs. The aim of this scoping review was to understand how elements of the cascade of care for TBI screening and treatment have been implemented in primary care settings globally and to consider these elements within the Australian context.
Methods
Eight databases were searched, including PubMed, Embase, CINAHL, Global Index Medicus, Scopus, Web of Science, ProQuest Dissertations & Theses Global and the Cochrane Library. The search strategy and MeSH headings were adapted to the database searched. Key words included ‘general practice’, ‘primary care’, ‘latent TB’, ‘TB infection’, ‘screening’ and ‘model of care’. Reference lists of publications on TBI screening and treatment were also screened for relevant articles.
Inclusion and exclusion criteria
Articles were screened from 1978, to align with the Alma-Ata Declaration of primary healthcare,15 up to February 2022. Studies were included if they were:
- based in primary care, including refugee and asylum seeker clinics that provide primary care for at-risk populations
- peer-reviewed articles and theses
- published in English, Bengali or Hindi.
Studies were excluded if they primarily covered:
- active TB disease and treatment
- TBI epidemiology, genetics, pharmacology, contact tracing or health economics
- participants who were primarily children aged under 12 years, pregnant women or healthcare workers
- settings other than primary care, such as prisons, hospitals or aged care
- co-morbid conditions or complications such as HIV, diabetes and autoimmune disease.
Study selection
After duplicates were removed, the titles and abstracts were screened by two independent reviewers (MC and LS) using Rayyan software (rayyan.qcri.org). Due to the large number of abstracts retrieved, MC and LS screened 50% of the articles independently and ensured a high rate of consistency in the inclusion/exclusion decisions before MC continued to screen the remaining abstracts. Throughout the process, the reviewers continued to work closely to ensure a consistent approach was achieved at all times. When reviewers did not reach a consensus, a third independent reviewer (MD) assisted with the decision.
Data extraction and analysis
Data were extracted for each eligible study for the country, year of study, study design and aim, results, and elements of the cascade of care investigated. Elements of the care cascade extracted included identification of at-risk persons, education of clients, eligibility for screening, clinical assessment by trained clinicians, application of a screening test, referral to a specialist for exclusion of active TB disease, diagnosis of TBI, commencement of treatment, follow-up and treatment completion (see Table 1).
Results
The search identified 12,536 records. Following the removal of duplicates, 7207 records were screened for eligibility by title and abstract. Full-text screening was undertaken for 82 articles, with eight articles meeting the criteria for this scoping review (Figure 2).
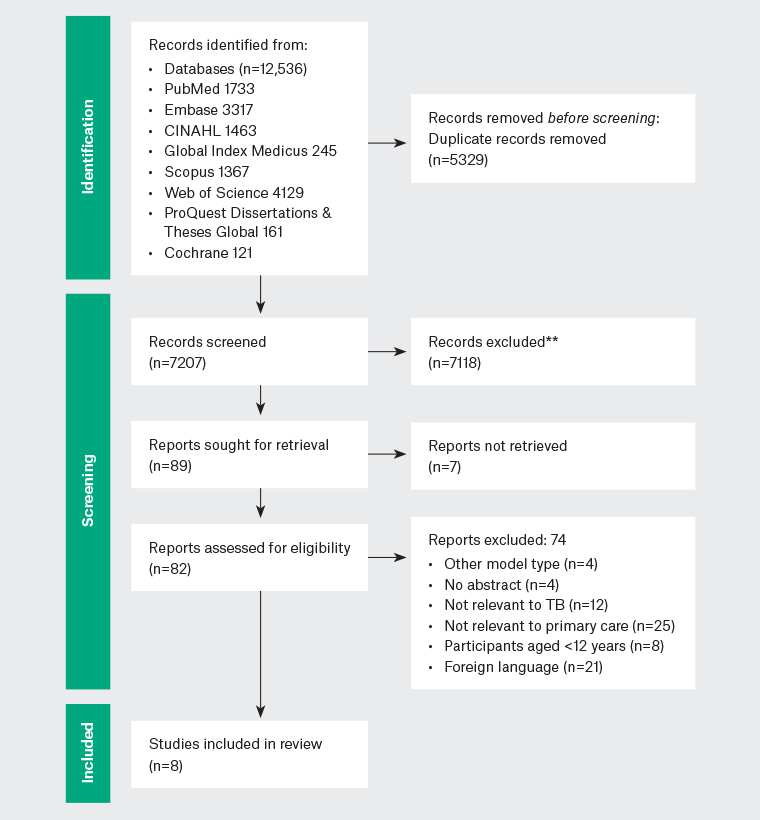
Figure 2. PRISMA flow diagram of decision making. Click here to enlarge
PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; TB, tuberculosis.
Study details
Details of the included studies are summarised in Table 1. Of the eight studies, one was a randomised controlled trial,6 one was a non-randomised trial,21 two were cross-sectional studies,18,19 two were cohort studies,17,20 and two studies used a mixed-methods design.8,16 Four were from the USA, two from the UK and one each were from Canada and Australia. Study participants included refugees,16,17 migrants,19,20 specific disadvantaged populations,18 patients of primary care clinics6,21 or a combination of the above.8 Eligibility for participation differed among studies. Some studies used a cut-off for the HBC rate in their research, such as >30 TB cases per 100,000 population16 and >40 TB cases per 100,000 population.19 Some studies specified age groups in the eligible criteria, such as those aged <40 years,21 >18 years,19 18–49 years16 and 18–50 years.8 One study was on newly arrived migrants19 and two were on refugees.16,17 Most studies were on adult populations, except Prater et al,20 which included people of all ages.
| Table 1. Study characteristics |
|
| Authors (year of publication; country) |
Year(s) of data collection |
Study design and aim |
Study setting and population |
TBI throughput (n) |
Primary finding |
|
Benjumea-Bedoya et al16
(2019; Canada)
|
January 2015 to October 2016
Ongoing program
|
Retrospective mixed-methods evaluation (patient outcomes data and qualitative interviews with clinic staff)
Aim: To evaluate patient uptake and outcomes of an integrated TBI screening and treatment program
|
A refugee health clinic in Winnipeg (BridgeCare Clinic)
Government supported refugees aged 18–49 years from countries with TB rate of >30/100,000 population per year
|
1010 refugees were seen at the clinic during the study period. 274 screening tests were ordered; of these, 158 (57.7%) were negative and 101 (36.9%) were positive
|
27 of 45 (79.4%) eligible TBI clients completed treatment
|
|
Carter et al17
(2017; USA)
|
August 2012 to April 2016
Ongoing program
|
Retrospective chart reviews
Aim: To assess the effect of a pharmacist-run clinic for TBI on TBI treatment completion rates in refugee patients
|
A primary care clinic for refugees (PCPC) refers patients to the pharmacist-run clinic
|
436 refugee patients were seen at a PCPC during the study period. At the initial screening, 135 were positive, 121 were diagnosed with TBI, and 103 were referred to the pharmacist-led TBI clinic
|
85 of the eligible 90 (94%) TBI patients successfully completed treatment
|
|
D’Lugoff et al18
(2002; USA)
|
1997–98
New program
|
Patient clinical data
Aim: To assess an academic health centre and local health department partnership designed to access a difficult-to-reach population and increase TBI screening and follow‑up treatment
|
Hispanic population in Baltimore at high risk of TBI
|
A convenience sample of 169 Hispanic migrants recruited through Spanish-speaking local organisations such as churches, apostolates and food outlets serving Latino cuisine. And all had TST administered
|
136 of the 169 (80%) had their TST read; 46 of 136 (34%) had a positive result; all 27 of 41 followed up (66%) were eligible for INH therapy and all commenced treatment
|
|
Griffiths et al6
(2007; UK)
|
June 2002 to October 2004
New program
|
Cluster randomised controlled trial
Aim: To assess an educational intervention for promotion of screening for TB (primary outcome – increased diagnosis of active TB; secondary outcomes – TBI diagnosed, BCG uptake, percent screened and TST undertaken)
|
50 of 52 GP clinics in the district of Hackney, London (a disadvantaged and ethnically diverse UK primary healthcare district)
|
44,986 patients in intervention and 48,984 patients in control practices, with 23,573 and 23,051 attending a registration health check at the intervention and control practices, respectively
|
57% and 0.4% of those at a registration health check were screened for TB at the intervention and control practices, respectively. Intervention practices had increased the diagnosis of TB (66/141 vs 54/157) and TBI (11/59 vs 5/68) in the intervention practices compared to the control practices
|
|
Hargreaves et al19
(2014; UK)
|
6-month period in 2013
New program
|
Cross-sectional study
Aim: To investigate whether a one-stop blood test approach to detect multiple infections would increase detection and treatment
|
Two GP practices attached to London hospital A&E in a high migrant area
Migrants aged 18 years or over who migrated <10 years ago and have lived at least 1 year in a country with >40 cases/100,000 population who attended a new patient health check were screened for eligibility and offered the blood test
|
1235 new registrations in the study period. 453 attended a new patient health check, of which 47 (10.4%) were identified as new migrants (≤10 years) in a population where 42.8% were foreign born
|
Of the 36/476, 6 agreed to participate; of the 6 that were diagnosed with TBI, 0 patients completed treatment
The number of migrants presenting for the screening was surprisingly low, reflecting barriers to care
|
|
Kunin et al8
(2022; Australia)
|
January 2017 to April 2018
Ongoing program
|
Convergent mixed-methods evaluation (cohort study with patients and focus groups with clinicians)
Aim: To describe and evaluate a TBI primary care model focusing on feasibility and barriers and enablers to its success
|
The model was piloted in one universal primary care clinic and one refugee-focused primary health service (MHRHW)
The eligible population were refugees and asylum seekers aged 18–50 years who screened positive for TBI and TB; 15 clinicians from primary care participated in the focus groups
|
Of the 65 TBI patients in the two clinics, 31 patients accepted treatment, of whom 15 and 16 were treated at MHRHW and a universal primary care clinic, respectively
|
Treatment completion was reached in 23 of 31 (74%) TBI patients who commenced treatment (collected at least 6 months’ medication according to pharmacy dispensing records)
|
|
Prater et al20
(2021; USA)
|
March 2015 to March 2017
Ongoing program
|
Retrospective cohort study consisting of chart reviews
Aim: To characterise the TBI care cascade at a community health cente and quantify successful completion of each step of the cascade
|
One community health centre within the Baltimore Medical System, which is an area with a large foreign-born population including refugees
All patients with a positive test for TB infection during the 2-year study period
|
Of the 418 individuals with positive test results, 266 were retained in care at the Baltimore Medical System, 214 of 266 were diagnosed with TBI, 157 of 214 (73%) were prescribed rifampicin for 4 months, and 32 of 157 (20%) were prescribed INH for 9 months; 141 of 157 (90%) initiated treatment
|
Of the 141 who initiated treatment, 119 (84%) completed the treatment; 20 of 32 (63%) completed 9 months of INH compared to 99 of 125 (79%) completing 4 months of rifampicin treatment
|
|
Steele et al21
(2014; USA)
|
October 2002 to January 2003
Ongoing program
|
Non-randomised prospective study
Aim: To determine the effects of computerised clinical decision support on screening rates for TBI
|
Two outpatient primary care clinics in the public healthcare setting, in Denver, Colorado
Patients from high TB burden countries who were aged <40 years
|
4135 patients were registered in the post-intervention phase, 73% had one CDC-defined risk factor, and 610 met the alert criteria for potential screening for TBI (birth in a high-risk TB country and aged <40 years)
|
Adherence to CDC guidelines improved significantly from 8.9% at baseline to 25.2% during the study phase
This study demonstrated that computerised clinical decision making and web-based documentation increased screening of high-risk patients for TBI
|
| A&E, accident and emergency departments; BCG, Bacillus Calmette–Guérin; CDC, Centers for Disease Control and Prevention; GP, general practitioner; INH, isoniazid; LTBI, tuberculosis infection; MHRHW, Monash Health Refugee Health and Wellbeing; PCPC, Penn Center for Primary Care; TB, tuberculosis; TST, tuberculin sensitivity test. |
Prater et al,20 Benjumea-Bedoya et al16 and Kunin et al8 examined the total programmatic effectiveness of the care cascade for TBI. These studies quantified the successful completion of the steps in a community health centrewith a large foreign-born population in Baltimore (USA),20 a primary healthcare facility for refugees in Winnipeg (Canada)16 and a population from diverse backgrounds including refugees in Dandenong (Australia).8 All other studies examined specific elements of the care cascade, such as outreach education,6 hard-to-reach populations,18 TBI treatment completion,17 TBI screening test strategy19 and improving internal programmatic practice.21
TBI cascade of care
Table 2 provides an overview of the elements of the cascade of care used in each study.
| Table 2. Elements of the TBI screening and treatment care cascade included in the studies |
| Authors |
Patient education and awareness raising |
Primary care staff training |
Initial assessment of risk for TBI |
Consent for TBI screening |
TBI screening |
Active TB exclusion |
Initiation of TBI treatment |
TBI treatment follow-up |
TBI treatment completion |
| Benjumea- Bedoya et al (2019)16* |
üü |
– |
üü |
ü |
ü |
üü |
üü |
üü |
ü |
| Carter et al (2017)17* |
üü |
– |
ü |
–** |
üü |
üü |
üü |
üü |
üü |
| D’Lugoff et al (2002)18 |
üü |
üü |
üü |
–** |
üü |
üü |
üü |
ü |
ü |
| Griffiths et al (2007)6 |
üü |
üü |
üü |
üü |
ü |
üü |
ü |
– |
ü |
| Hargreaves et al (2020)19 |
üü |
üü |
– |
üü |
üü |
ü |
ü |
ü |
ü |
| Kunin et al (2022)8* |
üü |
üü |
üü |
–** |
üü |
üü |
üü |
üü |
üü |
| Prater et al (2021)20* |
ü |
– |
– |
–** |
üü |
üü |
üü |
üü |
üü |
| Steele et al (2005)21* |
– |
üü |
üü |
–** |
– |
ü |
ü |
ü |
–
|
| TB, tuberculosis; TBI, latent tuberculosis infection; *, ongoing program; **, implied consent; –, not mentioned or not required; ü , mentioned; ü ü , detailed. |
Where an established healthcare policy or guideline existed for TBI screening for refugees and asylum seekers, the process was optimally streamlined in primary care.8,16,17,20 Where the broader local community was of interest, such as specific disadvantaged populations, identification and recruitment for TBI screening and treatment were more challenging. This was demonstrated in the US study, where identifying the at-risk Hispanic population in Baltimore City included collaborating with the Esperanza Center (formerly the Hispanic Apostolate), local churches conducting religious services in Spanish and restaurants serving Latin cuisine.18 Education of clients along with an initial clinical assessment was covered in seven of the eight studies.6,8,16–20 Education involved interpreters,16,17 bilingual staff18 and educational resources (eg information in multiple languages19 and pictograms for medication charts17); an exception was the study by Steele et al,21 where the aim was to test the feasibility of a computerised clinical decision-making tool. In Prater et al, which focused on TBI treatment, client education was described as being provided at a later point in the care cascade.20 Education and training of primary care staff in TBI screening and treatment was also critical for strengthening the TBI screening and treatment cascade, and was the primary aim of the randomised controlled trial undertaken in the UK.6
Screening tests were available within the primary care setting in seven of the eight studies. An exception was in the pharmacist-run model, where patients were referred to a pathology laboratory for the screening test.17 TBI screening tests varied based on available resources, feasibility and costs, as well as the screening policy of the existing TB control program in the country or jurisdiction. Three studies used the TST,6, 18,21 three used the IGRA test,16,17,19 and one used both the TST and IGRA.8 In Prater et al, the screening test offered was stratified by age (ie TST for children aged under five years and IGRA for others).20 Notably, the QuantiFERON Gold test was preferred for all studies applying the IGRA test and, for the TST, the Mantoux test was applied, except in the study by Griffiths et al, where the Heaf test was used.6 A one-stop test for screening of multiple diseases was investigated by Hargreaves et al, who concluded that such an approach might not be best placed in primary care as the at-risk population was hard to reach due to stigma, disadvantage and lack of awareness, making presentation to primary care a challenge.19
Exclusion of active TB disease was undertaken by specialists (ie chest physicians or infectious disease physicians) through referral from primary care and as deemed appropriate in all studies.
Treatment regimens were investigated and documented to varying degrees of detail in five of the eight studies.8,16–18,20 The studies by Griffiths et al6 and Steele et al21 focused on screening for TBI and TB and computerised clinical decision making, respectively, with limited focus on treatment. Although the type of treatment was not mentioned in the study by Hargreaves et al, they followed the UK National Institute for Health and Care Excellence (NICE) guidelines for treatment.19
Of six TBI patients identified in the study by Hargreaves et al,19 three did not attend specialist clinic appointments, two were not eligible for prophylaxis (being aged >35 years), and one was treated but experienced an adverse event and could not complete their treatment.
Of the five studies that reported on the type of treatment, isoniazid for nine months was the first line of treatment in three studies;8,16,18 in one study, either four months of daily rifampicin or nine months of daily isoniazid was prescribed19; and in the final study, rifampicin was the first line of treatment (where contraindicated, daily isoniazid for six to nine months was started).17 Side effects of isoniazid included gastrointestinal intolerance and liver toxicity, especially among those with alcohol dependence.16
Clinical follow-up and liver function tests ensured early detection of isoniazid side effects in two studies,8,16 with monthly follow-up of blood tests for alanine transaminase for liver function in only one study.16 Indications for treatment completion were six months of daily isoniazid in three studies8,17,18 or 180 doses of isoniazid in nine months or 270 doses of isoniazid in 12 months in one study.17 An interruption of treatment of more than 30 days meant treatment would need to recommence.17 For rifampicin, 120 doses in six months was considered treatment completion.17 Access through a co-located pharmacy20 and regular follow-up,8,16,17,20 pill counts and prescription refill dates along with a certificate of TBI treatment completion facilitated treatment adherence.17
Those who did not agree to medical treatment or had contraindications were offered biannual chest X-ray screening for two years for early detection of conversion to active TB.16
Medications were made available free of cost in the study by Kunin et al,8 whereas patients were responsible for the cost of their medication, with some insurance cover, in the study by Prater et al.20
Barriers to treatment initiation and adherence were investigated in the Canadian study and included concerns about side effects to the foetus during pregnancy and the difficulty understanding the need for medication, especially for younger patients.16 The nine-month course of isoniazid was perceived to be long and, hence, a barrier to treatment acceptance and completion. In addition, patient motivation due to the asymptomatic nature of TBI and the long treatment regimen were identified by Kunin et al as barriers to treatment initiation and adherence.8
Discussion
This scoping review identified that with a robust referral system and community mobilisation, TBI screening and treatment are feasible in primary care. The reviewed studies indicate that primary care has a place in TBI screening and treatment, with some studies concluding that primary care is well situated to deliver TBI screening and treatment, especially in the delivery of the following elements: education for clients, training of staff, application of screening tests, interpretation of results, commencement of TBI treatment, follow-up and completion of treatment. Some studies identified an increase in TBI screening rates; however, barriers in implementing TBI screening and treatment in primary care were also identified, with gaps in the delivery of some elements of the care cascade.
The broad elements of the cascade of care (Figure 1)5 include the identification of at-risk individuals, followed by education, application of a screening test (TST or IGRA); exclusion of active TB in screen-positive individuals; and commencement, follow-up and completion of TBI treatment. Underpinning the model of care is the assumption that TBI fulfils the eligibility criteria for population-based screening.22,23
For new migrants or individuals in the community who were born overseas in HBCs, identification and recruitment to screening programs were found to be challenging, as they tended to experience marginalisation or disadvantage and were, therefore, disinclined to present to primary care. Community mobilisation activities would be required to ensure eligible individuals present to primary care.
Nurse-run and pharmacist-run TBI screening and treatment models were shown to be effective,17,20 and are intuitively less resource intensive than physician-led models.20 However, a physician is necessary in the care cascade for clinical confirmation or exclusion of active TB disease, confirmation of TBI and recommendation of the appropriate TBI treatment for the patient. Specialist referral by general practitioners was universal in the care cascade. Partnerships or collaborative approaches with teaching organisations or government health departments were effective and represented an important element of the TBI care cascade model in primary care.8,18
A TBI definition, based on TST or IGRA reading along with a clinical assessment, was not provided in any of the reviewed studies. A study based in the Netherlands by Spruijt et al does provide a definition, although general practitioners were outside the model of care for TBI screening and treatment, and the screening doctors were either infectious disease physicians or TB/chest specialists in secondary care.24
Follow-up for early detection of side effects, ensuring adherence and completion of TBI treatment, seemed to be best achieved with a pharmacist-run model17 or trainee nurse-led models.18 A certificate of TBI treatment completion awarded to clients was an important incentive for adherence as it meant rescreening could be avoided if clients relocated within the USA, and the certificate could assist with citizenship.17
Future directions in the Australian context
In Australia, TBI screening and treatment would need to be examined and dovetailed in the context of in-migration and settlement policy, demography and trends, capacity and capability of primary care settings, support systems and resources. A partnership or collaboration with a robust referral system of specialist clinical staff will be essential. IGRA testing and rifapentine–isoniazid prescribing will need to be accessible for eligible community members in primary care. Community mobilisation activities will be critical, as they will enable and facilitate at-risk individuals and populations to present to primary care.
The Australian Government’s Communicable Diseases Network Australia developed a position statement in 2017 that indicates Australia’s political will, commitment and interest in the TBI screening and treatment strategy.25 However, more research is required in this area, especially in developing a model of care that best fits specific jurisdictions and regions.
Limitations
Although the models described in the studies were heterogenous and varied, the data available were useful to identify gaps in the available evidence in models of care in TBI screening and treatment. Although in some studies the care cascade was not sufficiently documented, there was substantive information to add value to the findings of this scoping review. Variations in characteristics were partly attributed to differences in each country or its jurisdiction’s governmental policies, priorities, organisational structure and funding, which limited the ability to conduct in-depth comparisons across studies and generalisability to the Australian general practice setting. The review was limited to articles published in English, Hindi and Bengali.
Conclusion
TBI affects individuals and populations from HBCs living in LBCs, for many of whom survival and settlement are prioritised over preventive care. Primary care was overwhelmingly acknowledged to be well suited to undertake TBI screening and treatment, with a robust referral or partnership strategy and effective systems. No one model of care exists that can be readily adopted by primary care in LBCs or within a country or its jurisdictions or regions, although identification and education of vulnerable populations, application of a screening test, follow-up, monitoring and completion of TBI treatment seem to be the elements of the cascade of care that can be delivered in primary care in the Australian context. Further research is needed to investigate the feasibility, acceptance and effectiveness of a model of care for the delivery of TBI screening and treatment targeting at-risk populations in the Australian context.
Australia could pioneer an evidence-based, patient-centred, targeted model of care for TBI screening and treatment in primary care to further reduce TB notification numbers, thereby facilitating progression towards TB elimination.