Discussion
BU can manifest as an ulcer or as a non-ulcerated skin lesion, such as a plaque, nodule or oedema. Diagnosis can be achieved with a dedicated
Mycobacterium ulcerans polymerase chain reaction (PCR) test performed on a wound swab. Swabs on non-ulcerated disease have a high false negative rate, and a PCR test should be performed on a tissue biopsy to confirm disease. Most cases are managed with prolonged antibiotic therapy – commonly a combination of oral rifampicin and clarithromycin or fluroquinolone (moxifloxacin or ciprofloxacin) – and wound dressings.
Buruli ulcer (BU), caused by Mycobacterium ulcerans, is an under-recognised, debilitating soft tissue infection, which typically causes chronic progressive non-healing ulcers. These lesions can result in severe cosmetic deformity, functional impairment and substantial financial cost and have psychological consequences.1 Infection is endemic to multiple countries worldwide, but the incidence is increasing in Australia. Endemic regions include Victoria, where BU has been called Bairnsdale ulcer, and regions of Far North Queensland, where it is known as Daintree or Mossman ulcer.2 In non-endemic regions, delays in patient presentation and diagnosis increase the complexity of treatment and lead to worse outcomes.3 This is important because one-third of patients who acquire BU in endemic regions are visitors who can return to non-endemic locations with infection.4,5 Therefore, it is important to continue to raise awareness about diagnosis and the management of BU, particularly for general practitioners (GPs), who are often at the front line of diagnosis.
Aim
This article provides Australian GPs with an overview of BU, including information about transmission, epidemiology, clinical features, diagnosis and management.
Transmission
Mycobacterium ulcerans is an environmental pathogen with no reported cases of human–human transmission.6 It has been proposed that infection might occur through direct human contact with environmental sources, such as contaminated water, soil or vegetation.7 The mode of transmission is not entirely clear, but evolving evidence suggests it is potentially a zoonotic disease. M. ulcerans DNA and RNA have been identified in high concentrations in the excreta of possums, and BU cases correlate geographically with areas where possum excreta are positive.8,9 It is postulated that infection spills over from the possum population, potentially through mosquito transmission to human hosts10,11 or through contact from environmental sources contaminated by possum excreta.9,12
Epidemiology
In Australia, clusters of infection occur in coastal Victoria, most notably along the Mornington and Bellarine Peninsulas, and in Queensland, near Rockhampton and Cairns.2 Since 2017, increasing numbers of cases have been acquired in large urban centres of Melbourne and Geelong.13 Since 2019, cases have emerged in New South Wales, with locally acquired cases reported in the coastal town of Batemans Bay.14 Acquisition is most likely to occur during summer, when mosquito colonies tend to peak and humans have greater exposure to the outdoors.5 As the incubation period of BU is four to five months, most cases are diagnosed in winter and spring.15
Clinical features
In Australia, the median age of diagnosis is 66 years, although patients of all ages can become infected.16 BU lesions often spare the soles of the feet and palms of the hands but are commonly located on the distal aspect of limbs, especially around large joints. Lesions are usually solitary, but 5% of patients have more than one site involved.17 BU skin lesions are painless in most cases and initially often manifest as non-ulcerative lesions, such as nodules, plaques or papules.18 Over time, these lesions ulcerate. They typically have a necrotic base, undermined edges and surrounding erythematous induration (Figures 1,2). Oedematous lesions, which clinically mimic cellulitis, account for 8% of cases and are the most rapidly progressive and destructive lesions (Figure 3).19 Secondary bacterial infection of the wound is uncommon, but it should be suspected in the presence of pain, fever and wounds appearing inflamed (Figure 3). The World Health Organisation has classified BU into three categories of severity: Category I, a lesion <5 cm; Category II, a lesion between 5 and 15 cm; and Category II, a lesion >15 cm in size or is otherwise clinically complex (eg multiple lesions, osteomyelitis or those involving critical sites).20
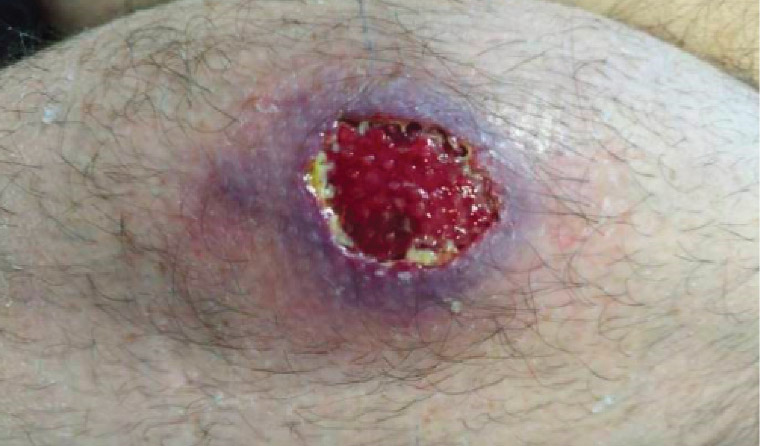
Figure 1. Right calf Buruli ulcer.
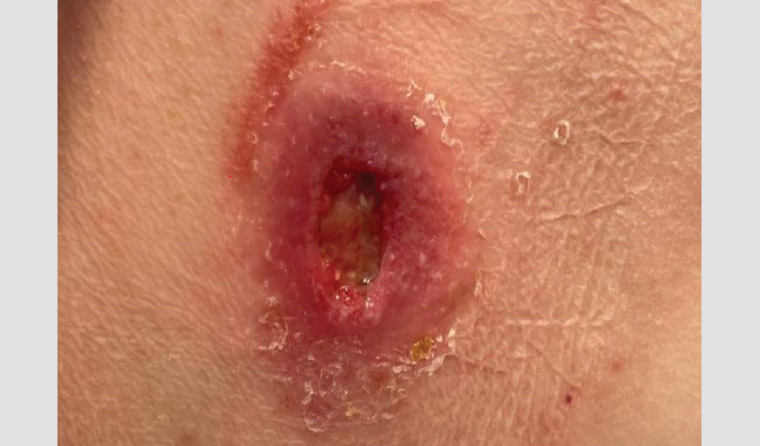
Figure 2. Right buttock Buruli ulcer.
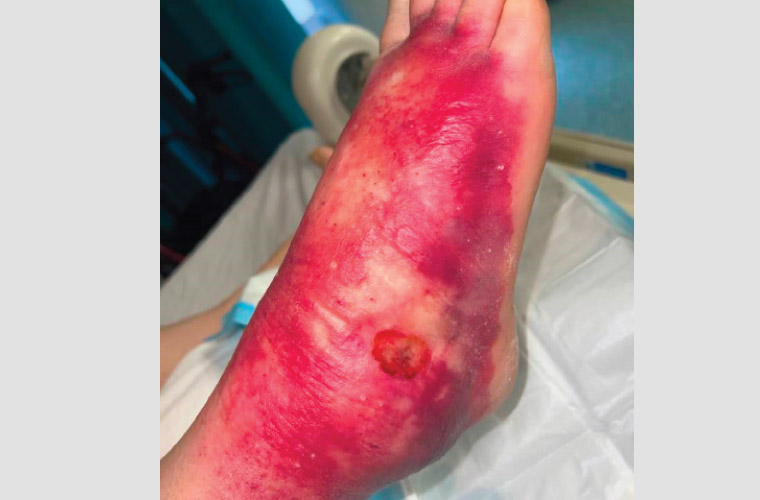
Figure 3. Oedematous form of Buruli ulcer with suspected superimposed bacterial infection.
Diagnosis
In BU, M. ulcerans nucleic acid detection by polymerase chain reaction (PCR) testing on a swab of the ulcer is the diagnostic test of choice due to its speed and accuracy.21,22 Culture is less sensitive18 and it can often take weeks to months for a result to return.23 Importantly, an M. ulcerans PCR swab needs to be specifically requested and sent separately to a culture swab, if performed. In ulcerative lesions, the circumference of the undermined edge of the ulcer should be swabbed and visible wound material should be observed on the swab itself.24 False negative tests can occur, and a negative PCR test does not exclude the diagnosis.25 This happens more frequently in children and lesions swabbed early on in development, which can lead to misdiagnosis or delayed diagnosis.21 When there is a high index of suspicion, repeat PCR should be performed, preferably with fresh tissue from a biopsy.21 Histology can also support diagnosis.26 In non-ulcerative lesions, because M. ulcerans exists subcutaneously, these lesions require biopsy for diagnosis.21
Management
Antibiotic therapy
Antibiotic therapy is the cornerstone of BU treatment and alone is highly effective.2 Consultation with an infectious disease physician is recommended. Patient expectations should be addressed prior to treatment, as most lesions persist after the completion of antibiotics, with BU known to take up to 12 months to fully heal after treatment.27 Approximately one in five patients develop a paradoxical reaction to antimicrobial therapy, with deterioration in the appearance of the lesion after treatment commences and occasionally development of a new lesion in separate parts of the body. Paradoxical reactions tend to occur more commonly in oedematous lesions and in people aged >60 years.28 Although the cure rate is high, it is important to monitor for signs of relapse after treatment completion.29 Risk factors for relapse include being obese, male and on immunosuppressive agents.30
Due to the potential for the development of antimicrobial resistance and to improve efficacy, combination oral therapy is recommended. First-line treatment in adults includes rifampicin 10 mg/kg to a maximum daily dose of 600 mg/day (adult and child), in combination with either clarithromycin 500 mg twice a day (child: 7.5 mg/kg twice a day to a maximum of 500 mg twice daily) or ciprofloxacin 500 mg twice a day (not recommended in children) or moxifloxacin 400 mg/day (not recommended in children) for a period of eight weeks. Dose reduction of clarithromycin and ciprofloxacin is needed if renal function is impaired.2,31
Practitioners should educate their patients about the side effects of long-term antibiotics, such as gastrointestinal upset, hepatotoxicity, hypersensitivity, Clostridioides difficile infection, antibiotic-associated diarrhoea and, rarely, neuropsychiatric side effects and peripheral neuropathy. All patients must be warned that it is normal to have red discolouration of bodily fluids due to rifampicin.32,33 Clarithromycin and quinolones can cause QTc prolongation, and a baseline electrocardiogram is recommended. Fluroquinolones are associated with tendinopathy, and caution is advised in older patients and those on corticosteroid therapy.2,34 Potential drug interactions are common, and patients should be advised not to start new medications, including over-the-counter and herbal medicines, without checking with their healthcare practitioner. Due to the risk of hepatotoxicity, alcohol abstinence is also recommended.
Surgical management
Surgery is not required to cure lesions but continues to have a role when medical therapy is not tolerated, when patient preference is for rapid wound closure in small ulcers, for debridement of necrotic tissue to improve wound healing, and in extensive ulceration when wound healing is felt to be significantly prolonged and to affect quality of life.2,35 Small lesions can be cured by surgery alone with wide margins, but large lesions benefit from adjuvant antibiotic therapy for four to eight weeks prior to surgery to reduce inflammation.36
Wound care
Good wound care is important to improve healing rates and prevent secondary infection. The general principles of wound care should be adhered to, including debriding necrotic tissue, reducing tissue oedema, and providing moisture and nutrition to the wound and protecting it from further soiling or trauma. Necrotic wounds benefit from the use of topical debriding agents, and those with copious exudate might require daily to alternate daily dressing changes with absorbable materials.37
Heat therapies
Thermotherapies have been used to cause local controlled hyperthermia of infected soft tissue in the setting of BU. In vitro, M. ulcerans is nonviable at temperatures >37°C.38 Markota et al hypothesise that heat therapy creates uninhabitable conditions for organism replication.39 Case series and phase 2 clinical trials using heat packs for an average of 10 hours per day for eight weeks showed the efficacy of heat therapy for BU.40 Heat therapy could be considered when antibiotic therapy and surgical management are not possible or desired.
Public health measures
Public health measures are limited by an incomplete understanding of disease transmission. In case control studies, preventive strategies that reduce mosquito bites through repellents and nets are associated with reduced risk of BU infection.41 Ensuring protective long-sleeved clothing is worn in endemic areas and proper wound care might also reduce acquisition risk.5,41 Future development of a vaccine for M. ulcerans might provide greater protection against infection.42,43
Key points
- Buruli ulcer is an important infectious differential of chronic non-healing skin lesions.
- Practitioners should develop a high index of suspicion in patients who visit endemic areas and be vigilant for new cases in previously non-endemic regions.
- An M. ulcerans PCR test is the gold standard for diagnosis, and in ulcerated lesions, the undermined ulcer edges should be swabbed. However, in non-ulcerated forms, tissue biopsy is required.
- Consultation with an infectious disease physician is recommended for management of BU.
- The mainstay of management is prolonged duration of a rifampicin-based antibiotic combination regimen and wound care, but surgical therapy can have a role in treating M. ulcerans.