Advanced melanoma, once a terminal prognosis, now has a 50% long-term survival rate, largely due to the use of new drugs, especially immune-checkpoint inhibitors (ICIs).1 Immune checkpoints are receptors in the immune system that can turn down a signal (inhibiting the immune response). The blockade of these checkpoints enables the body’s immune system to attack cancer cells more effectively. These ICIs are increasingly integrated into early stages of patient treatment, such as in the perioperative setting, playing a vital role in preventing recurrence.2 The introduction of these immunotherapies has significantly enhanced survival rates; however, they often entail considerable toxicities, highlighting the critical need for a synergistic approach between specialists and general practitioners (GPs) for the best patient care.
Aim
This article aims to provide GPs with a comprehensive overview of the evolving landscape in melanoma treatment. Focusing on advancements in early intervention, it serves as a guide for GPs, emphasising the importance of timely referrals to medical oncologists. The discussion encompasses novel therapies, immune-related adverse event management and ongoing trials, highlighting the collaborative role of GPs and oncologists in advancing melanoma care and improving patient outcomes.
Advanced melanoma: Where we stand today
Historically, patients facing an advanced melanoma diagnosis had a median overall survival (OS) of approximately six months;3 however, the landscape has dramatically shifted with the introduction of ICIs a decade ago. ICIs are antibodies designed to target different inhibitory pathways within the immune system, such as CTLA-4, PD-1 and LAG-3, stimulating immune activation against cancer cells (Table 1).
| Table 1. Three types of immune checkpoints targeted by specific blocking agents in cancer therapy |
| Abbreviation |
Immune checkpoint |
Blocking agent |
| CTLA-4 |
Cytotoxic T-lymphocyte-associated antigen 4 |
Ipilimumab |
| PD-1 |
Programmed cell death protein 1 |
Nivolumab, Pembrolizumab |
| LAG3 |
Lymphocyte-activation gene 3 |
Relatlimab |
The start of this revolution traces back to the approval of ipilimumab, a CTLA-4 inhibitor, a protein receptor that downregulates the immune system. Despite only 10% of patients treated with ipilimumab having a clinical meaningful tumour regression (termed ‘response’), approximately 20% of the treated patients were alive long term (and likely cured) compared to less than 10% prior to ipilimumab use, thus marking the beginning of the ICI era.4,5
Shortly thereafter, both pembrolizumab and nivolumab, PD-1 inhibitors, more than doubled the median OS (approximately 32 months compared to 16 months) and provided durable survival, and likely cure, for approximately 40% of patients.6 The attempt to synergise the effectiveness of anti-CTLA-4 and anti-PD-1 culminated in the phase 3 randomised study, CheckMate 067, where patients receiving the combination of ipilimumab and nivolumab had a response rate of approximately 60%, with 50% alive at 6.5 years.1 Although this is truly remarkable, this success came at the cost of severe autoimmune toxicities in up to 60% of patients (discussed below).6
Recent developments combing a new ICI, relatlimab (a LAG3 checkpoint inhibitor), with nivolumab doubled the progression-free survival (PFS) of patients compared to nivolumab alone (10 months vs 5 months).7 Importantly, this combination demonstrated a much more favourable safety profile than the ipilimumab/nivolumab combination.7 Formal comparisons between the two combinations have yet to be made.8
Beyond ICIs, nearly half of patients with melanoma have tumours that carry the BRAF mutation, a gene involved in a critical cell growth signaling pathway.9 Oral inhibitors of mutant BRAF and the downstream protein MEK (dabrafenib/trametinib, encorafenib/binimetinib, vemurafenib/cobimetinib) have led to rapid responses in almost all patients, and with little toxicity; however, unlike with ICIs, such responses are rarely durable.10–12
Drug treatment for early-stage disease: A paradigm shift
Recent achievements in the treatment of advanced melanoma are now influencing early-stage treatment, focusing on reducing the risk of recurrence after surgery. Patients with stage III melanoma, with regional lymph node or in-transit metastases, have a risk of recurrence after surgery as high as 65% within five years. Here, one year of postoperative (‘adjuvant’) therapy with anti-PD-1 agents (nivolumab and pembrolizumab) or BRAF/MEK inhibitors (dabrafenib/trametinib) roughly halves the risk of recurrence.13–15 Patients with stage II melanoma, who have thick primary tumours, but with no regional metastases, also have a high risk of reoccurrence, and also have disease recurrence reduced with adjuvant anti-PD-1 therapy.16–18 New adjuvant treatment strategies using individualised neoantigen therapy (ie a novel protein antigen arising from somatic mutations in cancer cells recognised as foreign by the immune system) with an mRNA vaccine (using the same technology as the mRNA COVID-19 vaccines) in combination with anti-PD-1 therapy is currently under investigation (clinical trial: NCT05933577), after promising early phase results.19,20
Neoadjuvant therapy (ie pre-surgery drug treatment) has even more impressive results.21 In particular, a pre- and then post-surgery treatment with pembrolizumab was shown to be superior to the standard post-surgery adjuvant-only treatment in stage III (and IV) patients, with a 20% reduction in the risk of recurrence.2 A promising clinical trial is ongoing (NCT04949113) to further compare neoadjuvant-combined anti-CTLA-4 and anti-PD-1 treatment with the standard adjuvant anti-PD-1 treatment, in the same population of patients.22 Beyond survival improvements, neoadjuvant therapy also provides a personalised response assessment at time of surgery, which enables individualised prognosis and management. This likely also has health economic and quality-of-life improvements. As such, neoadjuvant therapy is now standard care for clinical stage III melanoma.
Considering these advancements and the widespread availability of clinical trials and curative-intent therapies for localised melanoma, it is crucial for GPs to be aware of these medical therapies and play an active role in recognising and managing toxicities. Figure 1 outlines the current standard management algorithm for patients with localised resectable melanoma. Although great improvements have been made, not all patients benefit from current therapies, and more advances are required to improve outcomes. As such, clinical trials should still be considered for all patients with melanoma.
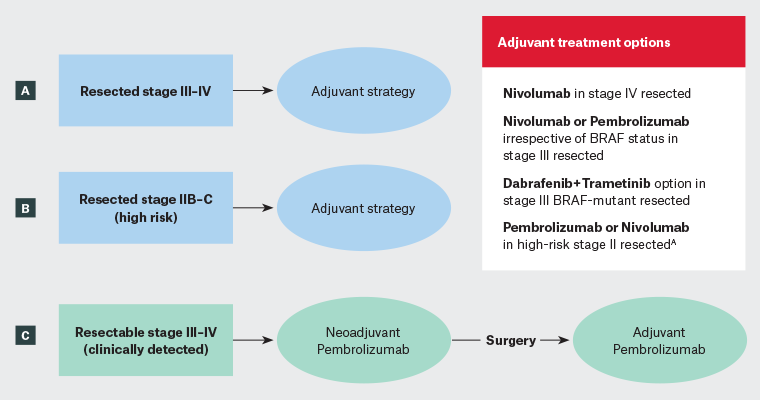
Figure 1. Algorithm of the current standard therapies for the management of resectable melanoma. ‘Resectable melanoma’ refers to a melanoma that can be surgically removed, typically indicating that the cancer has not advanced to a stage where surgical treatment is no longer an option. In this figure, we discuss high-risk resectable melanoma, where a neoadjuvant therapy (immunotherapy started before surgery) strategy could be considered. (A) Complete lymph node dissection for clinically occult stage III melanoma (ie with positive sentinel lymph node) is no longer required. These patients should receive 1 year of adjuvant therapy. For resected stage IV melanoma, nivolumab is the only studied agent. (B) Patients with thick melanoma, but without lymph node involvement (Stage IIB–C), are now eligible for 1 year of adjuvant therapy with pembrolizumab or nivolumab. (C) To receive neoadjuvant therapy, the patient should be referred to a medical oncologist (or to a specialised centre) before surgery to benefit from preoperative pembrolizumab.
AThese drugs are not yet listed on the Australian Pharmaceutical Benefits Scheme for this indication
Immune-related adverse events: Early detection is key
Immunotherapy with ICIs operates by blocking cell receptors normally involved in immune system suppression.23 The inhibition of these checkpoints boosts the patient’s immune system to target melanoma cells; however, this activation might also involve clones of self-reactive lymphocytes, leading them to attack other healthy organs or tissues, resulting in immune-related adverse events (irAEs), which can mimic autoimmune disease.24 As indications for ICIs are increasingly expanding, even in the earliest stages of melanoma, GPs play a vital role in the comprehensive care of cancer patients undergoing immunotherapy and require a clear understanding of irAEs and their management.25
Cutaneous, gastrointestinal, endocrine, pulmonary and musculoskeletal irAEs are relatively common, whereas cardiovascular, haematologic, renal, neurological and ophthalmic irAEs occur less frequently (Figure 2, Table 26,7,14,15,17,26,27).28 Although the majority of irAEs are mild to moderate, severe and occasionally life-threatening cases have been reported, with up to 2% resulting in treatment-related fatalities, varying by the specific ICI (or combination) used.28 Unlike chemotherapy-induced adverse events, side effects of immunotherapy are unpredictable in onset, can be difficult to detect and can affect any organ.25 Successful management relies on early identification, exclusion of differential diagnoses and prompt intervention using immune suppression (corticosteroids) or other immunomodulatory strategies.29,30
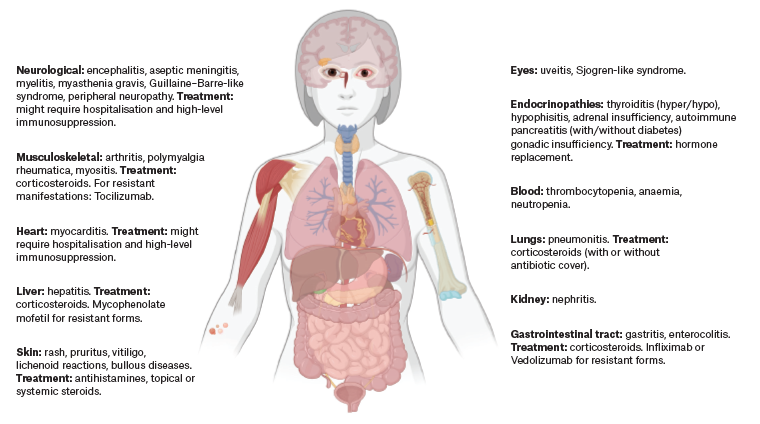
Figure 2. Overview of irAEs and their management. This figure illustrates the spectrum of potential irAEs affecting various organ systems in patients undergoing immune checkpoint inhibitor therapy. Key adverse events are depicted along with their respective treatments, ranging from corticosteroids to specific immunosuppressive agents. The visual representation emphasises the importance of multidisciplinary management for the diverse and potentially serious side effects associated with immunotherapy. Created with BioRender.com (BioRender, Toronto, Ontario, Canada). Click here to enlarge
irAEs, immune-related adverse events.
| Table 2. Rates of serious adverse events (grade ≥3) and specific toxicities associated with different immunotherapy regimens in main melanoma clinical trials |
| |
Rate of serious adverse events (grade ≥3) |
Endocrinopathies |
Gastrointestinal (grade ≥3) |
Pneumonitis or hepatitis (grade ≥3) |
| Nivolumab or Pembrolizumab6,7,14,17,26,27 |
15–20% |
Mainly hypothyroidism (10%) |
2–4% |
2% |
| Ipilimumab (3 mg/kg)6,26 |
20–30% |
2–4% of hypophysitis |
8% |
2–6% hepatitis |
| Nivolumab + Ipilimumab (3 mg/kg)6 |
60% |
17% hypothyroidism, 7–9% hypophysitis |
15% |
7% pneumonitis, 10% hepatitis |
| Nivolumab + Relatlimab7 |
20% |
18% thyroiditis, 4% hypopituitarism |
1% |
6% hepatitis, 4% pneumonitis |
| Anti-PD-1 monotherapy showed 15–20% overall serious adverse events, mainly hypothyroidism (10%), with additional occurrences of gastrointestinal issues (2–4%), pneumonitis or hepatitis (2%), and other symptoms (15–30% of low-grade arthralgia or pruritus/rash). Anti-CTLA-4 monotherapy, at a standard 3 mg/kg dose (as its toxicity is dose-dependent) showed a higher severe toxicity rate (20–30%), including hypophysitis (2–4%) and more significant gastrointestinal complications (8%). Combination Anti-PD-1 + Anti-CTLA-4 (3 mg/kg) therapy exhibited elevated rates (60%) of serious adverse events, encompassing diverse endocrinopathies, gastrointestinal problems, pneumonitis, hepatitis and hypersensitivity reactions. The new Anti-PD-1 + Anti-LAG3 combination regimen showed a mild frequency (20%) of serious adverse events. |
Patients with pre-existing autoimmunity undergoing ICI therapy do not appear to develop de novo irAEs at an increased rate.31,32 However, flare-ups of existing autoimmunity are common during ICI treatment.31,32 Education on irAEs is crucial, and patients should receive dedicated information from a medical professional, including informational booklets or reference cards.25
The most common and standardly managed toxicities are cutaneous (rash and itching) and rheumatologic (joint pain and arthritis) in nature.30 These are treated with symptomatic medications for milder forms (such as antihistamines and/or topical steroids), but might require corticosteroid therapy for more aggressive and resistant cases, necessitating specialist referral for steroid-resistant or specific dermatological manifestations (eg bullous diseases).30
Endocrinopathies are relatively frequent and are almost always permanent, managed with hormone replacement therapy. Hypothyroidism, affecting approximately 10% of patients receiving anti-PD-1 therapy, is the most common type and usually arises within the first few months of treatment, often diagnosed preclinically through routine monitoring.30 Some cases initially present with a short phase of hyperthyroidism, which might need treatment with beta-blockers and occasionally steroids if symptomatic.30 Hypophysitis, which most commonly causes isolated cortisol deficiency, can present subtly with symptoms such as fatigue, headaches and hypotension.30 It might escalate to adrenal crisis. GPs should maintain a high index of suspicion for such presentations, and consider early morning cortisol levels for diagnosis, coordinating closely with an oncology team for management. Treatment primarily involves steroid replacement. This condition is more common with the use of anti-CTLA-4 (ipilimumab), affecting 10% when combined with nivolumab.30 Another rare but important endocrinopathy is diabetes (termed checkpoint inhibitor-associated diabetes mellitus; CIADM), which affects approximately 1% of patients, and can mimic idiopathic type 1 diabetes but results in more difficult glycaemic control and almost always presents as ketoacidosis.30,33
Diarrhoea and enterocolitis are relatively common irAEs, with approximately 15% of patients developing severe symptoms, particularly if they are treated with anti-CTLA-4 therapy.30 It is crucial not to underestimate these symptoms, especially if they arise within the first months of treatment. Treatment for severe forms often requires systemic corticosteroids, infliximab or vedolizumab.30 This situation underscores the complexity of immune modulation in clinical practice, as it involves a delicate balance between activating the immune system to target cancer cells while simultaneously managing overactivation that leads to harmful autoimmune-like side effects.
Although very rare, potentially life-threatening toxicities can affect the cardiovascular or neuromuscular systems.30,34 Severe forms typically manifest within the first two cycles of treatment and might present with subtle symptoms like ptosis, fatigue and myalgia, leading to overlapping conditions such as myocarditis, myasthenia gravis and myositis.34 Other potentially fatal neurological toxicities include Guillain–Barré-like syndrome, encephalitis and aseptic meningitis.30
IrAEs constitute a heterogeneous group of manifestations that can potentially affect any organ or tissue. Moreover, these toxicities can also appear later, even after treatment cessation.30 GPs should be aware of their existence and be informed of the initiation of ICI therapy in their patients, and most importantly, should be able to communicate promptly with the oncology team about any potential or suspected toxicity (and vice versa). Although most irAEs are easily manageable, familiarity with even the rarest manifestations is crucial to avoid potentially fatal complications.
Regarding more severe irAEs necessitating systemic corticosteroid therapy, it is essential not to overlook the serious risks associated. Osteoporosis, adrenal suppression, hyperglycaemia, dyslipidaemia, cardiovascular disease, Cushing’s syndrome, psychiatric disturbances and immunosuppression are among the notable side effects, particularly when high doses are used for prolonged periods.35
Conclusion
As an increasing number of melanoma patients undergo drug treatments, particularly immunotherapy, prompt recognition of potential toxicities as a result of treatment and effective communication between GPs and the oncology team is crucial for both acute and chronic management.
Advanced melanoma, once considered universally fatal, has transformed into a disease with long-term survival rates. This change underscores the importance of maintaining regular health checks, including skin examinations,36 cancer screenings, cardiovascular risk indicators such as blood pressure and cholesterol. Additionally, managing both acute and chronic toxicities, including the side effects of immunosuppressive treatments like steroids, is crucial. This comprehensive approach to health is key to improving patient outcomes in the evolving landscape of melanoma treatment.
Key points
- Advanced melanoma, once terminal, now has a 50% ‘cure’ rate with immunotherapy.
- Systemic treatment has now shifted into early-stage melanoma via adjuvant and neoadjuvant therapies.
- General practitioners are crucial in referring newly diagnosed patients, as well as recognising and managing immune-related adverse events associated with immunotherapy and general comorbidities.
- Ongoing trials exploring novel strategies such as individualised neoantigen therapy and neoadjuvant immunotherapy show promise.