Melanoma overdiagnosis occurs when melanomas, not destined to cause morbidity or death in a patient’s lifetime, are identified and treated.
Aim
This study considers the causes and magnitude of melanoma overdiagnosis in Australia and of past and potential mitigating factors. We also present data raising the possibility of a putative benefit of overdiagnosis in Australia; namely, a reduction in excess deaths in the geographical areas where melanoma is diagnosed more frequently.
Discussion
The defined cause of overdiagnosis is treatment of melanomas that are slow-growing or otherwise biologically indolent, to the extent that they would not harm the patient.1 This definition specifically excludes ‘false-positive results; that is, a positive test in an individual who is subsequently recognised not to have cancer’.1 However, it is also proposed that overdiagnosis might result from incorrect histological labelling of naevi as melanomas (misdiagnosis),2 which results from the acknowledged poor inter- and intra-pathologist concordance, and therefore diminished reliability in diagnosis of melanocytic lesions, especially, but not only, of histologically borderline lesions.3
Although prospective diagnosis of biologically indolent melanomas is not possible with current technology, misdiagnosis of benign lesions as melanomas can be mitigated by actions that increase diagnostic specificity, reducing the number of benign lesions excised for each melanoma diagnosed; the ‘number needed to treat’ (NNT).2 Irrespective of whether excision of naevi, misdiagnosed as melanomas, increases overdiagnosis as it is defined, we argue that reducing the NNT will reduce true overdiagnosis, because some of the lesions spared excision would undoubtedly have met histological criteria for melanoma if excised.
Much has been published about the potential harms of overdiagnosis.1,4 These include costs of treatment and more frequent follow-up, treatment-associated morbidity including discomfort and scarring, and related issues of anxiety and psychological stress impacting quality of life. There are also financial burdens of work-time lost and potential difficulties obtaining insurance. More broadly, there is the opportunity-cost burden on the health system. To balance this, it is known that mortality and costs are strongly positively associated with the stage of disease at diagnosis.5,6 Although the quantum of overdiagnosis can be estimated for cancers such as breast and prostate, by comparing screened and unscreened groups, this is not possible for melanoma in Australia because population screening is neither recommended nor performed.5 One analysis of differences in lifetime risk between 1982 and 2012 estimated the rate of overdiagnosis of melanoma to be 58%, including 22% for invasive melanoma.7
Various strategies have been proposed to reduce overdiagnosis. A proposal by Welch et al to stop self-referral of pathology specimens by dermatologists and to stop population screening1 might have merit but is not relevant in Australia where neither of these practices are employed. The same study suggested using a minimum lesion size of 6 mm as a criterion for biopsy and to discontinue the use of diagnostic practices including dermatoscopy. Regarding lesion diameter, it is known that small-diameter melanomas, which are frequently feature‑poor, can be invasive with potential for adverse outcomes; for example, it was found in one Australian study that melanomas that were three or less millimetres in diameter and were more likely to be invasive than larger melanomas.8 Moreover, an argument by Welch et al that dermatoscopic features ‘correlate poorly and inconsistently with biologic behaviour’1 is misleading, relying on a single article that does not support that assertion; that is, ‘not assessing the biologic behaviour of any lesions in its study’.9
Accepting the inescapability of melanoma overdiagnosis, what can be done to reduce its adverse impact? The number of practising dermatologists in Australia is limited to 645 (approximately one per 40,000 population).10 Understandably, this creates accessibility problems, but with the higher metropolitan concentration of specialist services, rural patients in particular can be faced with the need to travel long distances to see a dermatologist or surgeon. These factors, compounded by the highest incidence of melanoma globally11 have led to melanoma management in Australia being increasingly undertaken by general practitioners (GPs), who now manage more melanomas than either dermatologists or surgeons.12
Responding to the inadequate preparation of GPs for skin cancer management,13,14 diverse providers, including the University of Queensland,15 the Skin Cancer College Australasia16 and HealthCert,17 have developed training programs. Consequently, there is now a cohort of highly trained, degree- and certificate-qualified GPs, whose numbers exceed those of Australian dermatologists, working alongside their generalist colleagues.14
The Skin Cancer Audit Research Database (SCARD) demonstrates the impact of GP subspecialisation, dermatoscopy use, education and work experience, on the reduction of NNT for melanoma. SCARD was established in 2007, to provide a lesion‑tracking and performance-reporting tool for clinicians and to investigate skin cancer management by harvesting data.18 A study on Australasian SCARD data from 2008 to 2010 showed that GPs subspecialised in skin cancer management, who also reported a higher use of dermatoscopy, excised half as many benign lesions for each melanoma detected, compared to their generalist colleagues (NNT 8.5 vs 17; P<0.0001).19 Another study from 2013 showed that a cohort of 21 GPs also involved in the earlier study (the majority of whom were now degree-qualified in the field of skin cancer), reduced their average NNT from 10.78 to 5.56 (P=0.0037),20 without increasing the percentage of melanomas that were invasive; a surrogate for stable diagnostic sensitivity.
Serial digital dermatoscopic imaging (SDDI)21 and the use of baseline total body photography22 improves specificity for melanoma detection, resulting in fewer excisions. In particular, SDDI identifies lesions with biologically indolent, non-progressive behaviour, which is precisely what is needed to prospectively avoid overdiagnosis. As explained, some of the spared lesions would predictably have been diagnosed as melanoma if excised and therefore their identification effectively reduces the true overdiagnosis burden. In vivo imaging modalities such as reflectance confocal microscopy also have the potential to assist selection of lesions for biopsy, but are currently limited by availability and cost. Until developments in the field of genetic and molecular markers enable prediction of metastatic potential, the overdiagnosis conundrum is predicted to persist.
We have previously reported that the Australian Cancer Atlas23 for (invasive) melanoma demonstrates that the geographic areas of highest incidence, and by extension, of maximum epidemiological overdiagnosis, also correspond to the areas of lowest excess deaths (Figure 1).14,24 To clarify, ‘excess deaths’ among a cohort of people are deaths due to a cancer within five years of diagnosis, above and beyond the number of deaths expected if that cohort of people had the same mortality rates as the general population.23
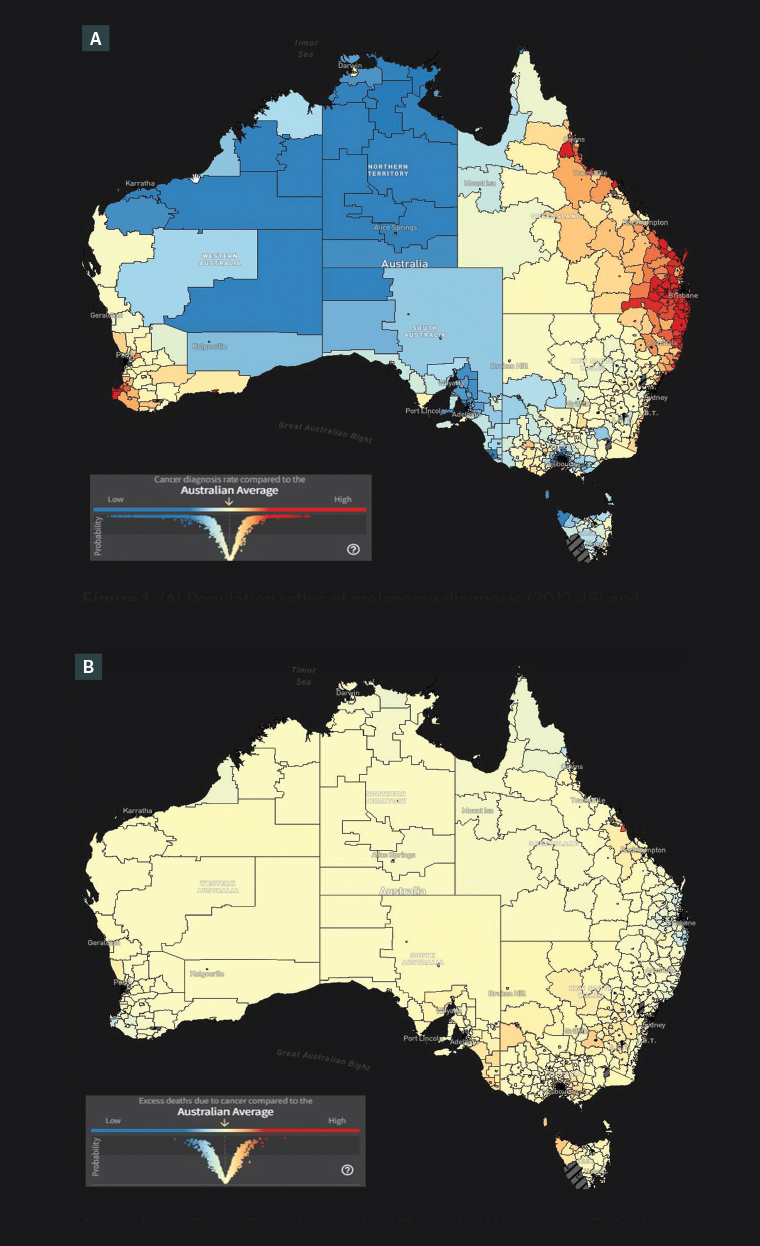
Figure 1. (A) Population ratios of melanoma diagnosis (2012–16) and (B) excess melanoma deaths within 5 years of diagnosis (2007–16) in Australia. Areas of maximum relative incidence compared to the Australian average (A, highlighted red, mainly at extreme right) correspond with areas of lowest excess deaths compared to the Australian average (B, highlighted darker blue, corresponding location).
Adapted from Cancer Council Queensland, Queensland University of Technology, Cooperative Research Centre for Spatial Information. Australian Cancer Atlas, version 02-2021. Cancer Council Queensland, Queensland University of Technology, Cooperative Research Centre for Spatial Information, 2021. Available at https://atlas.cancer.org.au, with permission from Cancer Council Queensland, Queensland University of Technology, Cooperative Research Centre for Spacial Information.14,23
To further investigate this, we explored how the correlation between cancer incidence and excess deaths seen in melanoma patients compares to that of patients with other cancer types. Incidence and excess deaths data of 20 cancer types across 2148 defined geographical areas were acquired from the Australian Cancer Atlas (Table 1).23 A statistically significant inverse correlation was observed for melanoma, with excess deaths decreasing with increased incidence (r = –0.5978, 95% CI: –0.6243 to –0.5699, P<0.0001), and this was the strongest inverse correlation observed among the cancer types (Table 1; Figure 2).
| Table 1. Correlation between cancer incidence and excess deaths within 5 years of diagnosis, for 20 cancer types |
| |
r |
95% confidence interval |
P-value |
| Melanoma |
–0.5978 |
–0.6243 to –0.5699 |
<0.0001 |
| Breast |
–0.4504 |
–0.4835 to –0.4160 |
<0.0001 |
| NHL |
–0.3945 |
–0.4296 to –0.3581 |
<0.0001 |
| Thyroid |
–0.3860 |
–0.4214 to –0.3494 |
<0.0001 |
| Myeloproliferative |
–0.3644 |
–0.4005 to –0.3271 |
<0.0001 |
| Prostate |
–0.3297 |
–0.3668 to –0.2914 |
<0.0001 |
| Myeloma |
–0.2698 |
–0.3086 to –0.2302 |
<0.0001 |
| Stomach |
–0.2174 |
–0.2574 to –0.1768 |
<0.0001 |
| Ovary |
–0.1184 |
–0.1599 to –0.07650 |
<0.0001 |
| Uterus |
–0.1087 |
–0.1503 to –0.06670 |
<0.0001 |
| Liver |
–0.1008 |
–0.1425 to –0.05877 |
<0.0001 |
| Brain |
–0.03696 |
–0.07913 to 0.005343 |
0.0868 |
| Pancreas |
0.05419 |
0.01192 to 0.09626 |
0.0120 |
| Cervix |
0.1754 |
0.1341 to 0.2161 |
<0.0001 |
| Kidney |
0.1997 |
0.1588 to 0.2400 |
<0.0001 |
| Bowel |
0.3165 |
0.2779 to 0.3540 |
<0.0001 |
| Lung |
0.3642 |
0.3270 to 0.4004 |
<0.0001 |
| Oesophagus |
0.4090 |
0.3732 to 0.4437 |
<0.0001 |
| Head/Neck |
0.6578 |
0.6331 to 0.6811 |
<0.0001 |
| Leukaemia |
0.7300 |
0.7096 to 0.7491 |
<0.0001 |
Cancer incidence (standardised incidence ratio) and excess deaths (excess hazard ratio) data of 20 cancer types across 2148 defined geographical areas within Australia was acquired from the Australian Cancer Atlas.23
The Pearson correlation coefficient (r value) was calculated, along with the associated confidence interval and P-value.
NHL, non-Hodgkin’s lymphoma.
Adapted from Cancer Council Queensland, Queensland University of Technology, Cooperative Research Centre for Spacial Information. Australian Cancer Atlas, version 02-2021. Cancer Council Queensland, Queensland University of Technology, Cooperative Research Centre for Spacial Information, 2021. Available at https://atlas.cancer.org.au, with permission from Cancer Council Queensland, Queensland University of Technology, Cooperative Research Centre for Spacial Information.23 |
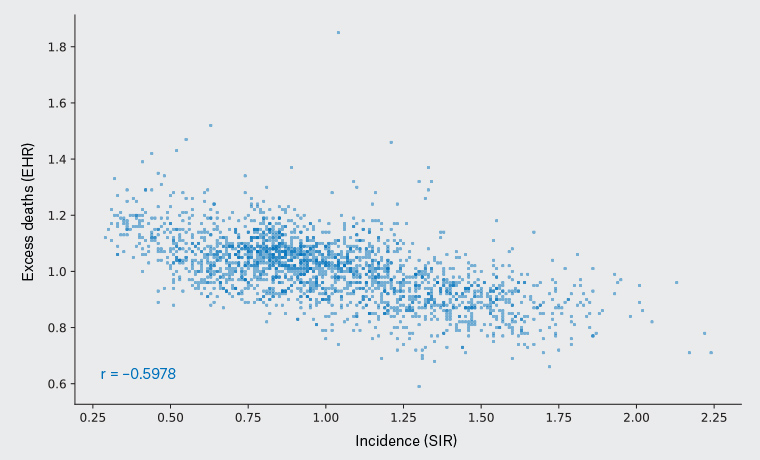
Figure 2. Melanoma incidence (X-axis) versus excess melanoma mortality (Y-axis) for defined geographical regions (individual data points). There is a significant trend of decreasing excess deaths in geographical regions with higher diagnosis rates of melanoma (Pearson correlation coefficient r=–0.5978, P<0.0001).
EHR, excess hazard ratio (the 5-year risk for a person in a defined area dying from melanoma proportional to the risk of a person in Australia dying from melanoma); SIR, standardised incidence ratio (the ratio of the number of cancers in a defined area to the number expected relative to the population of Australia).
Each data point is a Statistical Area Level 2 (SA2), defined as an area of residence, with a broadly similar size to postcodes, each representing a community that interacts together socially and economically.
Adapted from Cancer Council Queensland, Queensland University of Technology, Cooperative Research Centre for Spacial Information. Australian Cancer Atlas, version 02-2021. Cancer Council Queensland, Queensland University of Technology, Cooperative Research Centre for Spacial Information, 2021. Available at https://atlas.cancer.org.au, with permission from Cancer Council Queensland, Queensland University of Technology, Cooperative Research Centre for Spacial Information.23
Another study based on this Australian Cancer Atlas data found that 62% of the (all-invasive) melanomas were thin (one or less millimetres), and that relative proportions of thin, intermediate and thick melanomas were broadly consistent across areas of higher and lower overall incidence.25 The authors concluded that the overall incidence patterns were likely due to the underlying population risk profile rather than other factors; however, they conceded that further studies were necessary.
There are limited international population studies investigating diagnostic rates of melanomas relative to GP density,26,27 melanoma mortality related to rurality of location,28 and melanoma-specific mortality related all-cause mortality.29 And there have been no international studies found to have examined location-specific excess deaths versus incidence, comparable to the data presented in the Australian Cancer Atlas.
There has been a 27% decline in age-standardised, melanoma-specific mortality in Australia from 6.2 (per 100,000 population) at its peak in 2011, to 4.5 in 2019.30 Although this has been attributed both to preventative sun protection strategies5 and novel medications for advanced disease,31 neither of these can account for higher survival metrics with increasing incidence. This phenomenon could, however, be explained by an impact on survival of early diagnosis of melanomas, which would have actually resulted in death if not treated, and by an increase in the number of thin melanomas diagnosed with a lower potential for fatality. There are, however, other factors to consider. These include movement of people between geographical areas, and the fact that although overdiagnosis is apparently a cause of improved survival, we still do not know whether the improvement in survival is real, or alternatively, an artefact of overdiagnosis.
Conclusion
Melanoma overdiagnosis is a reality in Australia, being increasingly relevant in general practice where most melanomas are managed. We have shown how increased diagnostic specificity can reduce overdiagnosis. The impact on diagnostic specificity has been quantified for subgroups of GPs as a result of subspecialisation associated with a high level of dermatoscopy use, and with the likely impacts of education and experience over a time interval of three to five years. Finally, we have highlighted a reduction in excess deaths in geographical regions with the highest rates of melanoma diagnosis in Australia. Our speculation that early diagnosis of actual life-threatening melanomas in these geographical regions is positively impacting survival, while proposed, is not yet confirmed. Further research is planned.
Key points
- Melanoma overdiagnosis is diagnosis of melanomas destined to do no harm.
- Melanoma overdiagnosis can harm the patient and the community.
- Harmless melanomas cannot be prospectively identified.
- Reducing lesions excised for each melanoma discovered potentially reduces overdiagnosis.
- Melanoma survival is significantly higher in Australian regions with increased diagnostic rates.