The COVID-19 pandemic was a challenge for health systems worldwide. There were 13% fewer melanoma diagnoses in Victoria, Australia, in the first six months of the pandemic, compared with the same period in 2019.1 In the US, a study found that melanomas newly diagnosed during the pandemic had poorer prognostic markers such as high mitotic rates and greater incidence of ulceration.2 Numerous studies from across Europe reported an increase in the average Breslow thickness and a decrease in the number of melanoma diagnoses after the onset of the pandemic.3,4
Australia has the highest incidence of melanoma in the world.5 If a lesion is suspected to be melanoma, an excisional biopsy is performed whenever possible. Excisional biopsy provides prognostic information, such as Breslow thickness, which cannot be reliably obtained through a punch biopsy.6 If the diagnosis is confirmed, a wider excision is performed around the biopsy scar. For all patients with melanomas ≥1 mm in thickness or >0.8 mm thick with other high-risk pathological features, sentinel lymph node biopsy should be considered.7 If opting for a sentinel lymph node biopsy, it should be performed at the time of the initial wide local excision.7
In New South Wales (NSW), more than 50% of melanoma diagnoses are made by general practitioners (GPs).8 Most GPs refer thick melanomas to a surgeon or dermatologist for definitive management and perform wide local excision of in situ or thin melanomas themselves.9 GPs perform around 50% of wide local excisions in Australia, with dermatologists performing 16% and surgeons the remaining 34%.10
The mortality rate from COVID-19 in Australia was less than one-quarter the rate in the UK and US.11 COVID-19 policies varied widely across the country after the first case was reported in January 2020. NSW entered a strict lockdown on 31 March 2020, allowing residents to only leave their homes for essential reasons. These orders eased a few months later as case numbers dropped. However, Sydney re-entered a lockdown for four months between June and October 2021 after an outbreak of the Delta strain.12 In Victoria, Melbourne residents spent 263 days in lockdown between 2020 and 2021, which is the second longest time spent in lockdown of any city in the world.13 Restrictions in other states and territories were less stringent.13
Few studies to date have explored the impact of the COVID-19 pandemic on melanoma morbidity and management in Australia. Analysis of Medicare Benefits Schedule (MBS) data found a 29% decrease in skin checks in general practice in the second quarter of 2020.14 The Victorian Melanoma Service found a 48% reduction in new referrals during lockdown, and an increase in the average Breslow thickness of invasive melanomas from 2.06 to 2.70 mm.15
The aim of the present study was to investigate the impact of COVID-19 pandemic restrictions on melanoma management and characteristics in a public tertiary referral hospital in NSW, Australia.
Methods
This retrospective cohort study was conducted at Westmead Hospital, a public tertiary hospital in NSW, Australia. Data were collected on patients diagnosed with melanoma between 1 January 2019 and 31 December 2021.
This interval comprised the 16 months prior to the beginning of a strict lockdown in NSW (31 March 2020) and the 20 months after (pre-pandemic group: 1 January 2019 – 30 March 2020).
From 31 March 2020, NSW entered a strict five-week lockdown prohibiting people from leaving their home other than for essential reasons. Non-urgent outpatient clinics were cancelled or postponed, and there was increased uptake of telehealth in general practice. Further waves of the pandemic and periodic lockdowns meant there were changes to healthcare delivery throughout the rest of 2020 and 2021 (pandemic group: 31 March 2020 – 31 December 2021).
Data were collected in 2022 from electronic medical records. The inclusion criteria for this study were:
- adult patients with a new, histopathological diagnosis of cutaneous melanoma during the study period
- biopsy and/or wide local excision performed at Westmead Hospital.
Epidemiological data on age and sex, clinical data (time of biopsy, time of referral from GP or private dermatologist, time of wide local excision) and histological data (Breslow thickness, ulceration, subtype, spread to lymph nodes) were collected for all patients where available.
‘Biopsy’ refers to any biopsy type (ie excisional, incisional, punch or shave). ‘Wide local excision’ refers to surgical excision of the melanoma, aiming for clear margins, after the diagnosis has been confirmed through a biopsy.
A descriptive analysis of the data was performed. Categorical variables are presented as absolute numbers with percentages. Numeric variables are presented as the mean and standard deviation (SD) or median with interquartile range (IQR) depending on data distribution.
Univariate analysis of normally distributed continuous variables was performed using the independent samples t-test. Non-normally distributed continuous variables were analysed using non-parametric tests (Mann–Whitney U test). Univariate analysis of categorical variables was performed using the Pearson Chi-squared test. Those differences that had a P value <0.05 were accepted as statistically significant.
A multivariate analysis was not performed because there were no significant differences in the epidemiological or histological data variables before and during the pandemic when univariate analysis was performed.
R software (R Foundation for Statistical Computing, Vienna, Austria) was used for the statistical analysis.
The study was approved by the Human Research Ethics Committee of Westmead Hospital (HREC 2110-09).
Results
Patient demographics
Patient demographics are summarised in Table 1. The study sample comprised 177 melanomas (41.5%) in the pre-pandemic group and 249 in the pandemic group (58.5%). In total, 64.4% of the pre-pandemic population was male, compared to 63.5% of the pandemic population. Overall, the majority (53.1%) of patients were aged 61–80 years, with 57.6% of pre-pandemic patients and 49.8% of pandemic patients falling into this category. The mean age of patients in the pre-pandemic and pandemic groups was 67.4 and 68.2 years, respectively. There were no significant differences in the sex and age of patients before and after the onset of the pandemic (P=0.921 and P=0.601, respectively; Table 1).
| Table 1. Patient demographics overall and during the pre-COVID-19 pandemic and pandemic periods separately |
| |
Total |
Pre-pandemic period (1 January 2019 –
30 March 2020) |
Pandemic period (31 March 2020 –
31 December 2021) |
P value |
| No. patients |
426 |
177 (41.5) |
249 (58.5) |
|
| Age (years) |
|
67.4±14.8 |
68.2±15.4 |
0.601 |
| Age groups (years) |
|
|
|
0.337 |
| ≤40 |
29 (6.8) |
13 (7.3) |
16 (6.5) |
|
| 41–60 |
94 (22.1) |
35 (19.6) |
59 (23.9) |
|
| 61–80 |
226 (53.1) |
102 (57.6) |
124 (49.8) |
|
| >81 |
77 (18.1) |
27 (15.1) |
50 (20.2) |
|
| Sex |
|
|
|
0.921 |
| Male |
272 (63.8) |
114 (64.4) |
158 (63.5) |
|
| Female |
154 (36.2) |
63 (35.6) |
91 (36.5) |
|
Unless indicated otherwise, data are given as the mean±SD or n (%).
SD, standard deviation. |
Melanoma prognostic characteristics
Regarding melanoma prognostic and histological characteristics (Table 2), superficial spreading melanoma was the most commonly obtained histological subtype in both the pre-pandemic and pandemic groups, with an overall prevalence of 38.4% in the entire study cohort. No significant differences regarding histological subtypes were observed (P=0.909). The mean Breslow thickness per month prior to the pandemic ranged from 0.7 to 4.9 mm, compared with 0.7 to 3.3 mm during the pandemic (Figure 1). Nearly one-third of melanomas excised during the study period were in situ (31.3%), 30.1% pre-pandemic and 32.1% during the pandemic. The median Breslow thickness during the pre-pandemic period was 0.8 mm (IQR 0.0–2.3 mm) compared with 0.7 mm (IQR 0.0–2.5 mm) during the pandemic (P=0.767). When comparing only invasive melanomas, the median Breslow thickness was 1.5 mm in both groups, with an IQR of 0.7–3.5 and 0.6–4.0 in the pre-pandemic and pandemic groups, respectively. In the pre-pandemic group, 30.4% of melanomas were ulcerated, compared with 33.1% during the pandemic (P=0.724). Pre-pandemic, 31.9% of wide local excisions also included lymph node biopsy, compared with 36.6% during the pandemic (P=0.397). In cases where a lymph node biopsy was performed, 26.4% and 30.1% were positive pre-pandemic and during the pandemic, respectively (P=0.786).
| Table 2. Melanoma prognostic and histological characteristics overall and during the pre-COVID-19 pandemic and pandemic periods separately |
| |
Total |
Pre-pandemic period (1 January 2019 – 30 March 2020) |
Pandemic period (31 March 2020 – 31 December 2021) |
P value |
| No. patients |
|
177 (41.5) |
249 (58.5) |
|
| Subtype |
|
|
|
0.909 |
| Superficial spreading |
127 (38.4) |
56 (40.6) |
71 (36.8) |
|
| Nodular |
54 (16.3) |
22 (15.9) |
32 (16.6) |
|
| Lentigo maligna |
101 (30.5) |
41 (29.7) |
60 (31.1) |
|
| All others |
49 (14.8) |
19 (13.8) |
30 (15.5) |
|
| Breslow thickness (mm) |
|
0.8 [0.0–2.3] |
0.7 [0.0–2.5] |
0.804 |
| Breslow thickness (mm) of invasive melanomas only |
|
1.5 [0.7–3.5] |
1.5 [0.6–4.0] |
0.843 |
| Breslow thickness (mm) |
|
|
|
0.767 |
| 0 |
131 (31.3) |
52 (30.1) |
79 (32.1) |
|
| 0.01–0.50 |
104 (24.8) |
44 (25.4) |
60 (24.4) |
|
| 0.51–1 |
62 (14.8) |
29 (16.8) |
33 (13.4) |
|
| >1 |
122 (29.1) |
48 (27.8) |
74 (30.1) |
|
| Ulcerations present |
|
|
|
0.724 |
| Yes |
88 (32.0) |
34 (30.4) |
54 (33.1) |
|
| No |
187 (68.0) |
78 (69.6) |
109 (66.9) |
|
| Lymph nodes biopsied |
|
|
|
0.397 |
| Yes |
136 (34.6) |
53 (31.9) |
83 (36.6) |
|
| No |
257 (65.4) |
113 (68.1) |
144 (63.4) |
|
| Positive lymph nodes |
|
|
|
0.786 |
| Yes |
39 (28.7) |
14 (26.4) |
25 (30.1) |
|
| No |
97 (71.3) |
39 (73.6) |
58 (69.9) |
|
| Unless indicated otherwise, data are given as the median [interquartile range] or n (%). |
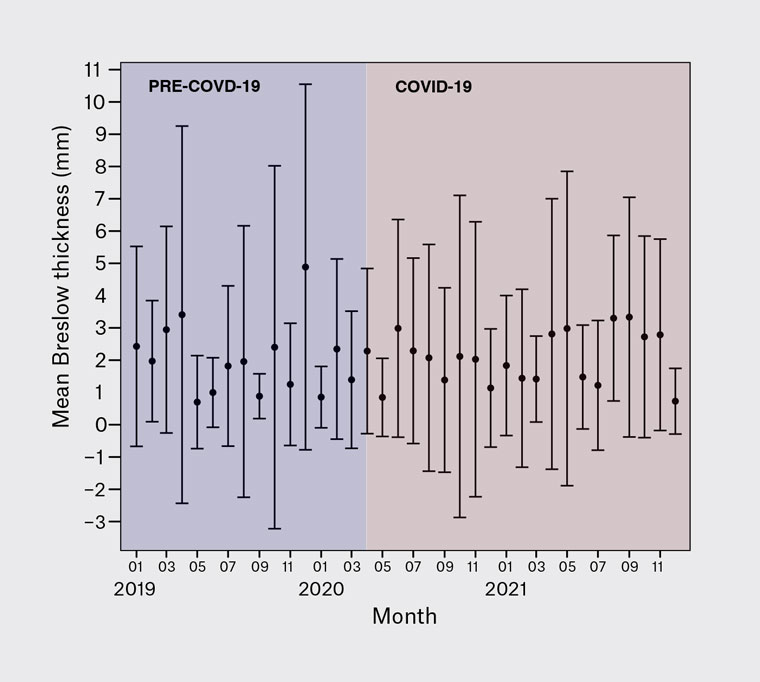
Figure 1. Monthly mean Breslow thickness (per month) of newly diagnosed melanomas before and during the COVID-19 pandemic. Error bars indicate the standard deviation.
Clinical data
During the study period, in the three years between 2019 and 2021, 426 cutaneous melanomas were diagnosed. Of all examined lesions, 177 (41.5%) were obtained prior to the pandemic and 249 (58.5%) were obtained during the pandemic. This equates to 11.8±4.5 and 11.3±4.3 diagnosed cases per month prior to and during the pandemic, respectively. Pre-pandemic, 78.5% of lesions were identified in the community by a GP or private dermatologist, with patients then referred for further management at Westmead Hospital. The remaining lesions (21.5%) were identified in outpatient dermatology or surgical oncology clinics at the hospital. During the pandemic, there was a decrease in the proportion of cases identified in the community (67.5%) and an increase of cases identified in hospital clinics (32.5%). This was a statistically significant result (P=0.016). In cases in which the biopsy was performed in the community prior to hospital referral, the median time from biopsy to referral for wide local excision was seven days in both the pre-pandemic and pandemic cohorts. The median time from referral to wide local excision decreased during the pandemic (Figure 2), from 50 days pre-pandemic to 39 days during the pandemic. This was a statistically significant result (P=0.013). The median time from biopsy to wide local excision was 48 days pre-pandemic, compared with 43 days during the pandemic. This was not a statistically significant result (P=0.239; Table 3).
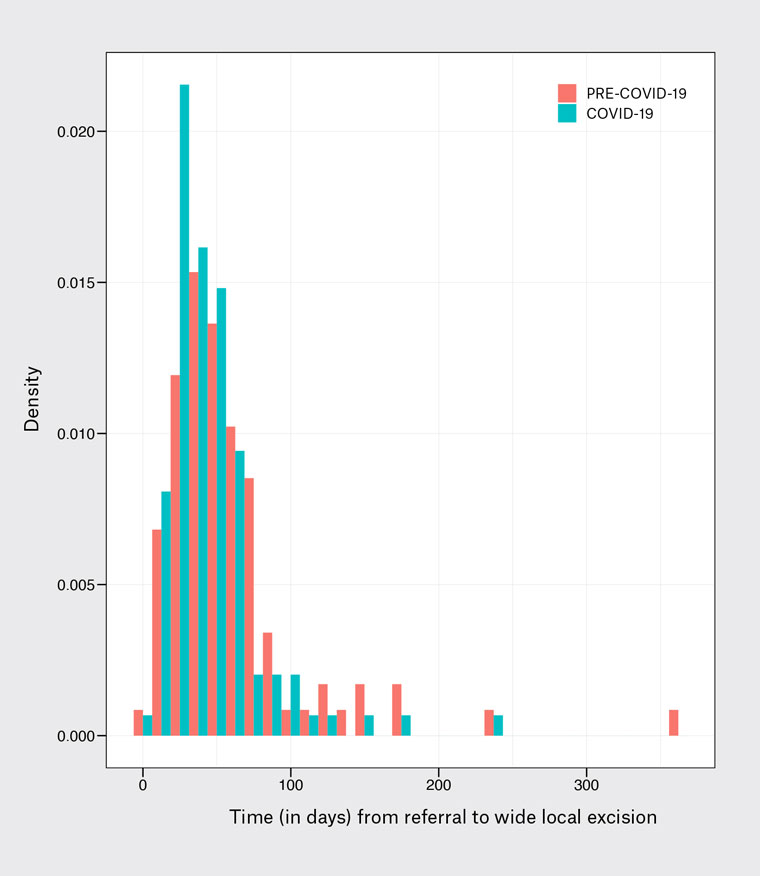
Figure 2. Density plot of duration (in days) from community referral (general practitioner or dermatologist) to wide local excision, before and during the COVID-19 pandemic. Median time from referral to wide local excision decreased during the pandemic
(50 vs 39 days; P=0.013).
| Table 3. Clinical data, including cases per month, setting of melanoma diagnosis and referral–biopsy excision intervals, overall and before and during the COVID-19 pandemic separately |
| |
Total |
Pre-pandemic period (1 January 2019 – 30 March 2020) |
Pandemic period (31 March 2020 – 31 December 2021) |
P value |
| No. patients |
426 |
177 (41.5) |
249 (58.5) |
|
| Cases per month |
|
11.8±4.5 |
11.3±4.3 |
0.745 |
| Lesion detection |
|
|
|
0.016 |
| In the community |
307 (71.1) |
139 (78.5) |
168 (67.5) |
|
| Within hospital clinic |
119 (27.9) |
38 (21.5) |
81 (32.5) |
|
| Time from biopsy to referral (days) |
176 |
7.0 [5.0–10.0] |
7.0 [5.0–9.0] |
0.887 |
| Time from referral to wide local excision (days) |
213 |
50.0 [33.3–69.8] |
39.0 [28.0–55.0] |
0.013 |
| Time from biopsy to wide local excision (days) |
313 |
48.0 [31.3–70.0] |
43.0 [33.5–62.0] |
0.239 |
Unless indicated otherwise, data are given as the mean±SD, median [interquartile range] or n (%).
SD, standard deviation. |
Discussion
In the short term, Australia’s stringent lockdowns were effective in preventing a massive loss of life from COVID-19.16 However, it is necessary to worry about the long-term impacts of these lockdowns.
There was a 29% decrease in the rate of skin checks in general practice in the second quarter of 2020 compared with the second quarter of 2019.14 This is in line with our findings, which showed a shift towards melanoma detection within outpatient hospital clinics as opposed to with GPs and private dermatologists. Prior to the pandemic, 78.5% of melanomas were identified in the community, compared with 67.5% during the pandemic.
The reduction in skin checks might be explained by the dramatic uptake in telehealth by GPs. Almost half (48.7%) of the Australian population used GP telehealth services between April and December 2020, compared with <2% in the same period in 2019.17 Australian general practice guidelines advise performing skin checks opportunistically, or if concern is raised about a specific lesion.18 Regular screening skin examinations are only recommended for patients that fit high-risk criteria.18 GPs cannot perform opportunistic skin exams, and patients cannot present suspicious skin lesions, during a telephone consultation. In addition, most Australian dermatologists believe it is inappropriate to perform skin checks via telehealth.19
Studies across the US and Europe have shown a decrease in melanoma diagnoses,2,20,21 an increase in Breslow thickness2,20–25 and a higher proportion of advanced-stage melanomas during the pandemic.2,20,26 A meta-analysis of several European studies found a 0.29-mm increase in mean Breslow thickness and a significantly higher rate of diagnosis of Stage III melanomas (odds ratio 1.58).27 Several explanations have been provided, including cancellation or delay of appointments due to lockdown restrictions2 and the shifting of healthcare resources from outpatient settings to inpatient care.22 The increased uptake of telehealth was suggested to have been detrimental to melanoma detection,25 and some patients might have avoided seeking care due to fear of infection.20,23 In Italy, 49% of dermatologists saw their practice activity more than halve during the pandemic period.28
In contrast, a study performed in a plastic surgery unit in Ireland found no difference in melanoma characteristics, including Breslow thickness and ulceration, between pre-pandemic and pandemic cohorts.29 That study also reported a statistically significant increase in melanoma diagnoses during the pandemic.29 The authors credited the swift reorganisation of services in their unit for their success: the unit set up a telemedicine service for skin cancer triage, and skin cancer surgeries were performed in non-COVID-19 provider centres and private hospitals.29
It is difficult to extrapolate overseas findings to Australia, because melanoma incidence and management vary widely. For example, the melanoma management pathway in Ireland is far less reliant on general practice. Over a 10-year period, only 8.5% of excisional biopsies in Ireland were performed in primary care, with the remainder performed by a specialist in a hospital setting.30
We found no significant differences in tumour characteristics before and after the onset of the pandemic. This is likely because melanoma clinics at Westmead Hospital were not postponed or cancelled. In fact, there were more dedicated melanomas clinics and more time allocated for melanoma excisions, made possible by the cancellation of eczema and psoriasis clinics. The increase in melanoma-related activity is reflected in the decreased waiting time for wide local excision. The time from a referral being made by a GP or private dermatologist to wide local excision at Westmead Hospital decreased by 11 days during the pandemic.
There were no further lockdowns in NSW in 2022, and COVID-19 restrictions were rapidly eased with non-urgent elective surgeries back to pre-pandemic levels by 7 March 2022.31 The results of the present study show that melanoma care in NSW was not detrimentally affected by COVID-19. Given the extremely poor prognosis of melanomas diagnosed after metastasis,32 it is a great achievement to have avoided diagnostic delays.
Limitations and opportunities for further research
The main limitation of this study is that the data were taken from a single centre. Another drawback is that tumour staging was not one of our outcomes. Finally, our study is subject to the limitations inherent to a retrospective study. Further research should be conducted in 10 or 20 years to assess any longer-term impacts, and a similar study should be conducted in other Australian states, particularly Victoria, where melanoma diagnosis is known to have been affected by the onset of the pandemic.15
Conclusion
In this study, we found no difference in Breslow thickness or rates of ulceration of newly diagnosed melanomas before and after the onset of the pandemic. In fact, we observed reduced waiting times for wide local excision of melanomas diagnosed in the community. The prioritisation of melanoma care during the pandemic at Westmead Hospital (NSW, Australia) has resulted in no apparent delays in diagnosis and treatment, providing a framework for handling future instances of unprecedented healthcare upheaval.