Median arcuate ligament syndrome (MALS) refers to the external compression of the coeliac plexus by the median arcuate ligament (MAL) with resulting symptoms.1 Compression alone is a common radiological finding in approximately 25% of the population; however, only 1% of the population have corresponding symptoms.2 Diagnosis can be difficult due to the wide differential for abdominal pain, specifically in the younger population. The clinical presentation can include postprandial abdominal pain, nausea and vomiting, food fear, constipation or diarrhoea, and weight loss.1
The MAL is the fibrous edge of the diaphragmatic crura, which passes over the aorta at the level of the first lumbar vertebral body, superior to the origin of the coeliac axis (Figure 1).3 In some patients, a low diaphragmatic insertion of this ligament from an anomalous fibrous diaphragmatic band can compresses the coeliac artery.1 Chronic compression by this ligament can lead to hyperplastic intimal changes of the coeliac artery.1 This might progress to cause stenosis or complete arterial occlusion, along with post-stenotic dilation and coeliac artery aneurysms.1
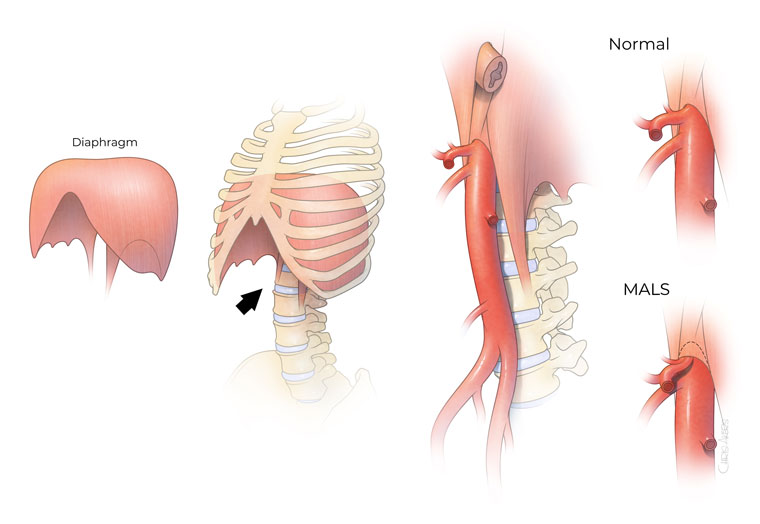
Figure 1. Illustration of median arcuate ligament syndrome (MALS). Patients experience severe compression of the coeliac artery, the first large branch from the aorta, as it enters the abdomen. This compression is typically worse during deep expiration.
Reproduced from The University of Texas Health Science Center at Houston (UTHealth). Median arcuate ligament syndrome (MALS). UTHealth, 2022. Available at https://med.uth.edu, with permission from The University of Texas Health Science Center at Houston.3
Pathophysiology
The pathophysiology of MALS is unclear, and multiple theories have been discussed in the literature.1,4 It is thought that the compression of the coeliac artery can cause foregut pain and ischaemia during increased demand when patients eat.4 This is somewhat contrary to the understanding that symptoms of chronic mesenteric ischaemia rarely occur secondary to isolated coeliac occlusive disease due to the rich collateral network of the mesenteric vessels.1,5 However, a prospective study in 2006 found that a positive gastric tonometry should be used to identify patients who would benefit from MALS release, suggesting that severe coeliac artery compression can result in ischaemic symptoms.6
Some postulate that a steal phenomenon occurs due to significant collateral circulation causing blood to be diverted away from the midgut to the foregut following meals, resulting in midgut ischaemia and pain.4
Further theories include that overstimulation of the coeliac plexus causes significant vasoconstriction, which results in ischaemic abdominal pain and the entrapment of the coeliac ganglion, altering gastric myoelectrical activity, impairing antral motility and causing neurogenic pain.4
Who is affected by MALS?
The prevalence of MALS remains unclear, which is partly due to its variable clinical presentation.1 There is a tendency towards women (4:1), and the median age is between 30 and 50 years.1 Cases have also been reported in paediatric populations.1,4 Furthermore, the literature describes an emerging theme of anxiety and mood disorders being higher in those with MALS.1,7
Primary care assessment
The primary presenting symptom of MALS is abdominal pain. Determining a diagnosis for chronic abdominal pain can be challenging due to the broad differentials and extensive work-up required, along with the significant effects on the patient’s quality of life. It can be an expensive burden both for the healthcare system and patients.8 Many patients are left with debilitating symptoms without a specific diagnosis, which can cause significant psychological distress.8
MALS occurs most commonly in young women. They describe abdominal pain, weight loss, bloating, nausea and vomiting, which can be related to eating and drinking or be exercise induced. A thorough history should be taken as to whether they have had previous abdominal surgery (eg diagnostic laparoscopy or cholecystectomy) and whether they have any other symptoms of autonomic disorders (eg postural orthostatic tachycardia syndrome).4
On physical examination, patients are classically slim with no significant abdominal pain on palpation. An epigastric bruit increased with expiration might be present.4 There are no known approaches to elicit the symptoms for diagnosis.
MALS does not have universally accepted diagnostic criteria.1 However, it is a diagnosis that remains one of relative exclusion and is typically the result of extensive investigations to exclude more common, alternative causes of abdominal pain.1,4
Recommended work-up includes targeted blood tests (full blood count and liver function, renal function and lipase/amylase tests), urinary culture, biliary ultrasound, and upper and lower endoscopies with Helicobacter pylori testing and food allergy testing, including coeliac disease. Progression to multidetector computed tomography (CT) and gastrointestinal motility testing might be indicated.1 If these tests provide abnormal results, appropriate referral should be made to confirm an alternative cause of abdominal pain.1 If the above investigations are normal, along with a finding of coeliac artery compression, a diagnosis of MALS should be considered and the patient would benefit from multidisciplinary input, including from a gastroenterologist and a vascular surgeon.
Specialist assessment
Suspicion of MALS on history and radiological evidence is sufficient to refer on to a surgeon. However, to avoid delay to patient care, ruling out more common pathologies, the above investigations and gaining gastroenterology specialist opinion are recommended. In Australasia, MALS is primarily diagnosed and managed by vascular surgeons, with interventions offered by general and vascular surgeons as well as interventional radiologists and pain specialists, depending on the patient’s needs.
Evidence of coeliac artery compression alone on CT is not diagnostic of MALS. CT can be used to evaluate the structural elements of the coeliac artery and the MAL, as well as highlight evidence of post-stenotic dilation or aneurysms.1,4 Features of coeliac compression on a CT include focal narrowing of the proximal coeliac artery with a characteristic hooked appearance (Figure 2). This ‘hooking’, along with the absence of calcified plaques, helps differentiate MALS stenosis from atherosclerotic narrowing.1,5,9 CT allows for the assessment of the other mesenteric vessels, collateral supply along with potential aneurysmal changes within the collateral networks, and other causes of abdominal pain. Its limitation is that it is not a dynamic investigation, which is an important factor when diagnosing MALS.
Duplex ultrasound, performed by an experienced vascular sonographer, can provide accurate measurements of coeliac artery compression during rest, inspiration and expiration.10,11 Dynamic testing can also be performed by vascular surgeons via digital subtraction angiography, demonstrating narrowing or change in the coeliac artery during respiration.1
Further diagnostic tests are still being evaluated, such as gastric exercise tonometry.5 If the gastric fluid is more acidic post exercise, this suggests significant coeliac artery compression or ischaemia.4 Reilley et al demonstrated improved outcomes in patients who undergo coeliac decompression when they have coeliac compression and positive gastric tonometry testing compared to those who have a negative tonometry.6
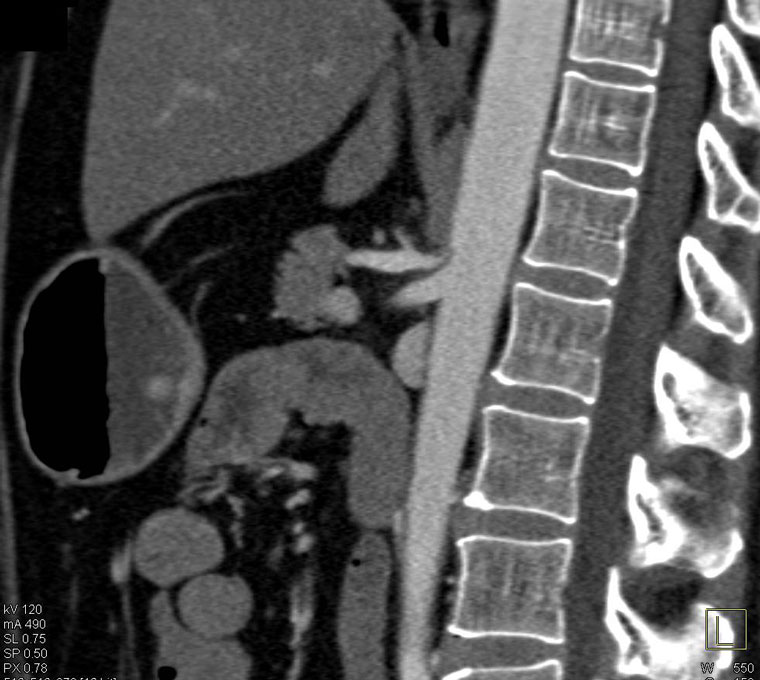
Figure 2. Classic hooked appearance of median arcuate ligament compressing the proximal coeliac artery, as seen on computed tomography angiography.
Treatment
If appropriate, intervention for MALS requires a multidisciplinary approach, including vascular surgery, general surgery, pain specialists and interventional radiology.
Coeliac plexus block is a non-surgical option for relief from MALS that can also aide as a diagnostic test.1,4,12 Coeliac plexus block involves an interventional radiologist or a pain specialist injecting local anaesthetic or a neurolytic agent directly into the coeliac plexus with imaging guidance. If the patient receives transient relief from intervention, this further supports a MALS diagnosis and might encourage surgeons to proceed to surgery.13
The surgical treatment of MALS includes decompressing the artery and performing a coeliac ganglionectomy.1,4 Decompression of the coeliac artery was traditionally performed through a laparotomy but is now more commonly performed via laparoscopically or robot-assisted laparoscopic release.13 In some cases, however, coeliac artery reconstruction might be indicated.
Endovascular stenting or angioplasty alone is deemed ineffective for MALS, as the extrinsic compression of the MAL might cause stenosis, fractures or migration of the stent. However, endovascular therapies have been used in patients with persistent stenosis of the coeliac artery post decompression.1
Unlike chronic mesenteric ischaemia caused by atherosclerosis, antiplatelet and cholesterol lowering tablets are not usually indicated for MALS. However, if performing coeliac artery reconstruction or coeliac artery angioplasty for recurrent stenosis post coeliac decompression, these medications are recommended to prevent neointimal hyperplasia.
On reviewing the literature, we have proposed a diagnostic and treatment pathway (Figure 3).
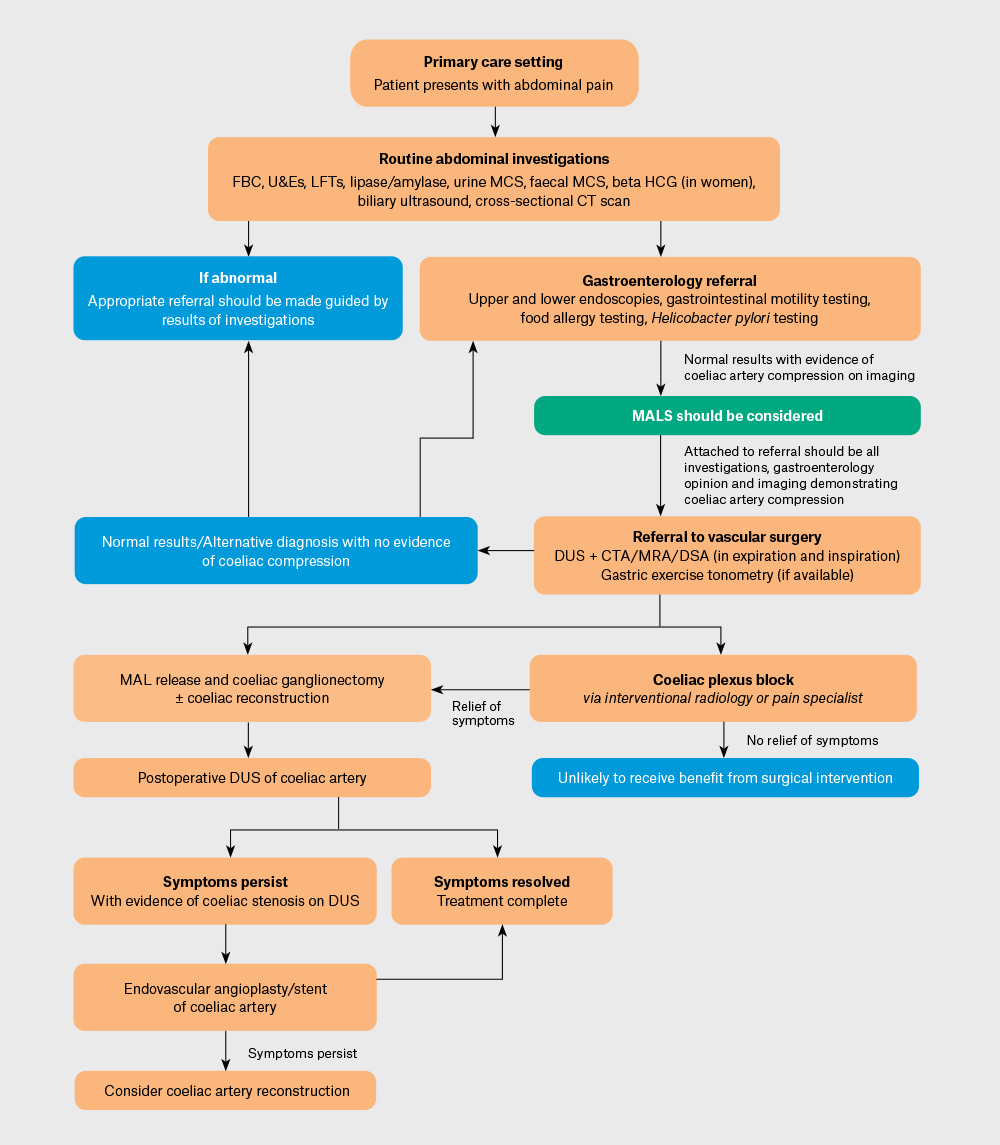
Figure 3. Proposed median arcuate ligament syndrome (MALS) work-up and treatment pathway.
CT, computed tomography; CTA, computed tomography angiography; DSA, digital subtraction angiography; DUS, Doppler ultrasound; FBC, full blood count; HCG, human chorionic gonadotropin; LFTs, liver function tests; MAL, median arcuate ligament; MALS, median arcuate ligament syndrome; MCS, microscopy, culture and sensitivity; MRA, magnetic resonance angiography; U&Es, urea and electrolytes.
Conclusion
MALS is a rare cause of abdominal pain that lacks defined diagnostic criteria. Those affected by MALs are often young, and it can cause significant morbidity. Testing in the primary care setting, as outlined in this article, is recommended to exclude other causes of abdominal pain and allow for subsequent referral. Due to the challenges in both diagnosis and treatment of MALS, multidisciplinary care involving general practitioners along with subspecialists is vital for successful outcomes. Those with MALS who have appropriate work-up, diagnosis and management have been found to have good outcomes in case series. Further consensus on diagnostic criteria and management is required. Regardless, an increased awareness of MALS would permit earlier consideration and investigation of the syndrome as part of a diagnostic work-up for patients with symptoms of abdominal pain and weight loss not attributable to more common conditions.
Key points
- MALS is rare, and diagnosis can be challenging, with only 25% of the population having radiological compression and only 1% of the population having corresponding symptoms.
- MALS is a diagnosis of exclusion and is typically the result of extensive investigations to exclude more common, alternative causes of abdominal pain.
- Postprandial and post-exertional abdominal pain in young women without an alternative diagnosis should undergo investigation for MALS via Doppler ultrasound, computed tomography angiography and, if available, gastric
- The pathophysiology of MALS is unclear but is likely an interplay between coeliac artery and coeliac ganglion compression.
- Coeliac plexus block, open surgical or laparoscopic MAL release and coeliac ganglionectomy have been found to be effective. However, the durability of symptom relief is variable.