Melasma, also known historically as chloasma or the mask of pregnancy, is an acquired skin hyperpigmentary disorder and is common among adult women. Melasma is characterised by brown or dark-brown pigmentation symmetrically involving the face: cheeks, forehead, nose, upper lip, chin and jawline. Figure 1 shows melasma in a woman and in a man. Melasma may start with macules that later become large patches with ill-defined borders and no pruritus. Based on the depth of pigment infiltration as determined using a Wood lamp, dermatoscope or histology, melasma is divided into epidermal, dermal and mixed (combined epidermal and dermal) types.1,2
Although the prevalence of melasma globally has been reported to be 1% based on data from Western countries, its prevalence is actually much higher, especially in women with skin of colour, namely those with Fitzpatrick phototypes III–V (ie those of Asian, Middle Eastern, Mediterranean African and Hispanic/Latin American descent), commonly affecting those aged 30–50 years.3–5 In this population of women, the actual prevalence of melasma has been reported to range from 9% to 40%, with higher rates among South Asian and Southeast Asian women.3–6 Aboriginal and Torres Strait Islander and Polynesian women are also affected, although no exact data have been established for these groups. Men can also be affected by melasma, but this is rare, with a male to female ratio of 1:9.4 In women, melasma can cause psychological morbidity, with aesthetic concerns and low self-esteem affecting quality of life. Thus, it is important to know how to manage melasma at the primary care practice (PCP) level when it is encountered.
Common differential diagnoses for melasma are listed in Table 1.5,6 Melasma can readily be diagnosed clinically based on the distribution and characteristics of pigmentation, as well as skin type or ethnicity, and a biopsy is rarely required. The cause of melasma is complex and multifactorial, and the precise aetiopathogenesis remains largely unknown. However, the risk factors listed in Box 1 are related to the development of melasma,1,5,7,8 and are important to identify and address.
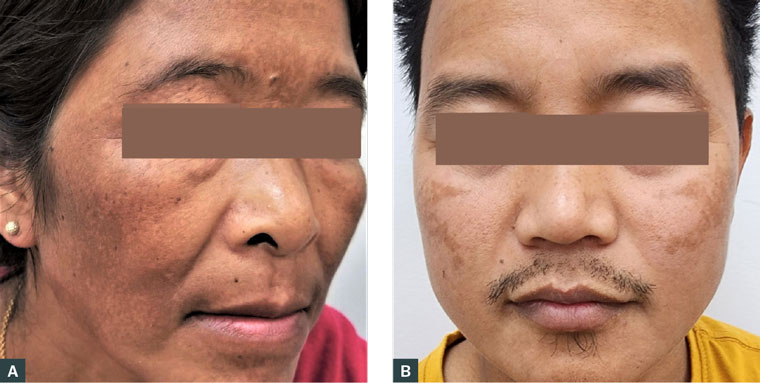
Figure 1. Melasma in (a) a woman and (b) a man.
| Table 1. Differential diagnoses for facial hyperpigmentary conditions |
| Condition |
Characteristics |
| Melasma |
- Reproductive age but mostly in ages 30–50 years
- Brown or dark-brown facial hyperpigmentation macules and patches in Fitzpatrick skin phototypes III–V (skin colour). No pruritus
|
| Postinflammatory hyperpigmentation |
- Any age and any site with prior inflammatory erythema in the area
- (eg prior contact dermatitis [pigmented cosmetic dermatitis] that
- can result from sensitising chemicals in cosmetic products)
|
| Drug-induced pigmentation/photosensitivity |
- Any age
- Blue or slate-grey pigmentation over the face, limbs, trunk
- and mucosa
- Tetracyclines, antiepileptics, sulphonamides, antimalarial and non-steroidal anti-inflammatory drugs are common culprits
|
| Actinic lichen planus (pigmented form) |
- Adults
- Pigmented erythematous patches or plaques over sun-exposed sites (face, neck and dorsum of hands)
|
| Solar lentigines |
- Fair-skinned individuals (Fitzpatrick skin phototypes I–III)
- Discrete, well-demarcated, pigmented macules over sun-exposed sites (face, neck and upper limbs)
|
| Discoid lupus erythematosus |
- Typically in young adult women
- Scaly erythematous patch or plaque over the face and scalp; look for autoimmune-associated manifestations
|
| Frictional melanosis |
- Adults
- At any site, resulting from excessive and repeated mechanical stimulation to skin (actively or passively rubbing/friction)
|
| Naevus of Hori |
- Adult Asian women
- Blue-grey to grey-brown patchy and spotty pigmentation on bilateral cheeks and less often on the forehead, nose and eyelids
- The condition is often misdiagnosed because it may resemble or coexist with melasma
|
| Poikiloderma of Civatte |
- Adult women
- Linear telangiectasia, erythema, mottled hyperpigmentation symmetrically on sun-exposed areas (cheeks, neck and upper chest)
|
| Box 1. Risk factors related to melasma |
- Light exposure: sunlight and visible/artificial light, especially cumulative exposure
- Genetic predisposition (20–50% positive family history)5,7
- Hormonal: hormonal contraception and pregnancy (15–50% of pregnancies)7
- Photosensitive medications (eg minocycline, doxycycline, non-steroidal anti-inflammatory drugs, antiepileptics, cytotoxics, psychotropics) and the use of some cosmetics
- Thyroid disorder (claimed to be, but needs further robust evidence)8
|
Management
Melasma treatment aims to diminish pigmentation by halting the proliferation of melanocytes, and the formation and degradation of melanosomes. There are several treatment modalities for melasma, as outlined below.
The first step, adopting general skin care protection measures, is always important. This includes reducing risk factors for the development of melasma, such as avoiding sun exposure and the use of hormonal contraceptives (if possible), phototoxic medications and certain cosmetics. Because sunlight can enhance pigmentation, sun protection during outings, including wearing a wide-brimmed hat or using an umbrella and the use of broad-spectrum tinted sunscreens (SPF ≥30), is recommended. Cosmetic camouflage is also a useful option.
Regarding specific treatment for melasma, there are both pharmacological and non-pharmacological options.
Pharmacological treatment
The wide range of pharmacological treatment options for melasma, including topical and systemic therapy, is detailed below.4,5,9
Topical treatments
Triple combined topical therapy consisting of hydroquinone (HQ), a potent lightening agent, with retinoid and corticosteroid is recommended as first-line therapy due to its efficacy and non-invasive nature compared with other modalities.5,9,10 Examples of triple combined topical therapy include Kligman’s formula (5% HQ + 0.1% tretinoin + 0.1% dexamethasone) or Tri-Luma (Galderma Laboratories LP, Fort Worth, TX, USA; 4% HQ + 0.05% tretinoin + 0.01% fluocinolone acetonide), which should be applied once daily over the lesion for four weeks and the melasma then reassessed. Treatment will take three to six months and adverse effects are minimal and may include skin irritation, erythema and post-irritant dyspigmentation. It is important to halt or cease an HQ-containing regimen if there is significant irritation because it may lead to postinflammatory dyspigmentation or ochronosis. The inclusion of a low-potency corticosteroid in the combined form is to counter or minimise such irritation, and to lessen some degree of hyperpigmentation. Retinoid works by reducing melanin synthesis, enhancing the penetration of other ingredients and improving skin tone from keratinocyte/epidermal turnover.1,10 HQ should not be used during pregnancy because its absorption through the skin is significant.11 The use of topical retinoids is also discouraged during pregnancy, albeit systemic absorption is reported to be negligible.12,13 These topical forms are currently not listed under the Pharmaceutical Benefits Scheme in Australia, although Tri-Luma has been approved for use in the US by the US Food and Drug Administration (FDA). In Australia, this form of combined topical treatment can be prescribed by customised script and made up by a compounding pharmacy. Practitioners can create a script for the combined topical form in practice software (Setup>Custom preparation) to print out when required.
Given Kligman’s formula can be modified with various ingredients and strengths, low- to medium-strength topical corticosteroids such as hydrocortisone 1%, betamethasone valerate 0.02% or triamcinolone acetonide 0.02% can be considered as interchangeable if dexamethasone 0.1% and fluocinolone acetonide 0.01% are unavailable in Australasia.14 Maintenance treatment with twice weekly (or appropriate frequency) application is recommended in view of easy relapse after stopping the treatment.
Other topical forms include single-agent treatments such as HQ (2–5%), azelaic acid (5–20%), kojic acid (1–2%), cysteamine cream (5%), ascorbic acid (5–15%), niacinamide (2–5%), tranexamic acid (TXA; 2–5%), glutathione (2%), tretinoin (0.05–0.1%) and corticosteroid (mild to mid-strength). Of note, combined treatment or the addition of an adjuvant generally offers better efficacy and effectiveness.1,4,9,10 A list of agents useful for the treatment of melasma, along with their mechanisms of action and side effects, is provided in Table 2.1,5,15
| Table 2. Topical agents used in the treatment of melasma with their mode of action and adverse effects |
| Agent |
Mechanism of action |
Adverse effects |
| Hydroquinone (2–5%) |
- Inhibits melanin synthesis by inhibiting the
enzyme tyrosinase
- Causes necrosis of melanocytes
|
Irritation and onchronosis, especially with high doses and/or extended use |
| Retinoid (tretinoin; 0.05–0.1%) |
- Rapid loss of the pigment via epidermopoiesis
and increased epidermal/keratinocyte turnover
- Reduces tyrosinase activity
|
Irritant dermatitis and photosensitisation |
Corticosteroid
(low potency) |
- Pigmentation fades due to reduced melanogenesis
and anti-inflammatory effect
|
No significant effects from low potency |
| Azelaic acid (5–20%) |
- Inhibits tyrosinase on aberrant melanocytes
- Anti-inflammatory effect
|
Irritation, dryness and pruritus |
| Kojic acid (1–2%) |
|
Irritation |
Ascorbic acid
(vitamin C; 5–15%) |
- Antioxidant: chelates copper ions, which serve
as a cofactor for tyrosinase activity
|
No significant effects from topical application |
| Niacinamide (2-5%) |
- Reduces melanosome transfer
- Anti-inflammatory properties
- Anti-ageing effects
|
No significant effects from topical application |
| Tranexamic acid (2–5%) |
- Disrupts melanin synthesis by blocking binding
of plasmin/plasminogen to keratinocytes
- Shrinks the dermal vasculature
|
Erythema, scaling, dryness and irritation |
| Cysteamine cream (5%) |
- Inhibits tyrosinase, halting melanin production
|
No significant effects from topical application |
| Glutathione (2%) |
- Decreases tyrosinase activity
- Proposed to be an anti-oxidant and reduce melanogenesis
|
No significant effects from topical application |
Systemic treatments
Among the systemic treatments available for melasma, namely oral TXA, carotenoids (lutein/zeaxanthin), glutathione and Polypodium leucotomos extract, TXA has been widely studied, including in randomised controlled trials, and has been shown to have favourable effects.16,17 TXA, which is mainly used for heavy menstrual bleeding, may be cost-effectively prescribed by general practitioners in Australasia. The proposed oral dosage is 250 mg twice daily or 500 mg daily for three to six months.16–18 In cases of severe melasma (extensive areas with dermal involvement) or melasma recalcitrant to topical therapy, a combined oral TXA with topical regimen has been reported to deliver better outcomes.3,17 The benefits of TXA in melasma treatment outweigh unlikely minor side effects (headache, epigastric pain, hypomenorrhoea and a theoretical risk of thrombosis), given the utilisation of a lower dosage.1,17
Non-pharmacological treatment
Non-pharmacological treatment modalities for melasma are generally beyond the capacity of general practitioners. These modalities include microneedling, chemical peeling, dermabrasion, light-based therapy (intense pulsed light, broadband light, pulsed dye laser), and laser therapy (Q-switched [QS] ruby, QS-Nd:YAG, ablative and non-ablative fractional lasers); unpredictable or mixed results have been reported from these treatment forms.4–6,19 Non-pharmacological treatment modalities are not cheaper than the pharmacological regimens described above and are not free from adverse effects either, such as erythema, paradoxical dyspigmentation, post-laser scar and even relapse. Currently, picosecond laser (1064 mm) is claimed to be effective with improved side-effect profile, but robust studies are needed to confirm this.20,21 Non-pharmacological treatment modalities should be reserved for patients for whom conventional treatment has failed or according to patient choice, with referral to a dermatologist or dermatologist-preferred specialist laser technician being warranted.
Melasma in pregnancy
Melasma in pregnancy may be transient and generally improve after delivery.1 Topical TXA, azelaic acid and kojic acid (Category B2) are favoured for the treatment of melasma during pregnancy if the patient wishes to be treated. To note, more novel treatments are expected to emerge, because no satisfactory treatment for melasma has been established to date. Recurrence or refractoriness to treatment is not uncommon for this chronic disorder. Melasma may clear up spontaneously, but its duration is unpredictable and depends on an individual’s risk factors and the severity of the lesions.
Conclusion
In the management of melasma, PCPs can play a vital role in initial assessment and treatment. Although dermatological expertise is necessary for sophisticated treatment forms, PCPs can effectively initiate treatment for melasma using combined topical therapies (with or without adjunct oral TXA) and follow-up. Melasma that is refractory to topical treatment or a patient’s choice for advanced therapy would warrant referral to a dermatologist.
Key points
- Melasma is a common skin condition among women, causing psychological morbidity and affecting quality of life.
- PCPs can manage melasma with topical treatment.
- Triple combined topical therapy (HQ, retinoid and corticosteroid) can be prescribed and obtained from a compounding pharmacy if ready-made preparations are unavailable.
- Referral to a dermatologist is warranted for advanced forms of treatment that are beyond the capacity of PCPs.