Familial hypercholesterolaemia (FH) is a genetic disorder affecting 1 in 250 people and contributes to premature cardiovascular disease (CVD).1 Failure to diagnose FH before middle age can result in up to 50% of affected males and 30% of affected females developing premature ischaemic heart disease.2 However, patients can achieve an average life expectancy if FH is detected and treated early.2 Treatment involves lifestyle interventions, management of other comorbidities and lifelong cholesterol-lowering medication. FH is largely underdiagnosed in Australia, with more than 90% of cases undetected.3,4
General practitioners (GPs) are optimally placed to screen for FH,5 and screening has been shown to be cost-effective.6 A thorough health and family history is central to establishing risk. However, GPs are often time-constrained and miss recording essential family history data in the patient record.7,8 Patients at risk might also not have a long-term GP to provide continuity of care, and therefore evolving strong familial risk might not be identified.9 A clinical diagnosis of FH can be made based on the Dutch Lipid Clinic Network Criteria, an algorithm focusing on family history, physical examination and low-density lipoprotein (LDL) levels. A recent Australian study by Brett et al extracted data from 15 practices containing 200,000 patients and identified 147 previously undetected cases of FH.10 This is likely an underestimation, because research has shown missing CVD risk factor data in >50% in patient electronic medical records (eMRs) in general practice,11 and family history is poorly recorded in the relevant data fields. Furthermore, LDL levels are routinely unavailable for patients aged <45 years because lipid testing is not recommended among the general population for this age group.12
This study addressed these limitations by developing, implementing and evaluating a targeted digital FH screening tool using patient self-reporting of medical and family history. This is the first known Australian study trialling an electronic self-screening intervention focused on increasing FH case detection and ultimately reducing the incidence of avoidable CVD.
Methods
Ethics, practice recruitment and consent
Ethics approval was obtained through the University of Notre Dame Australia Human Research Ethics Committee in May 2022 (Reference 2021-165S). General practices were recruited by convenience sampling. Eligible practices needed to use appointment management software programs with text message capacity (eg AutoMed, HotDocs). Each general practice provided written consent for patients to be invited to participate. Data collection took place between June and October 2022.
Patient eligibility, recruitment and consent
The study flow is outlined in Figure 1. Eligible patients were those aged 18–60 years who had a booked face-to-face GP appointment. Patients were prompted to screen by two strategies. First, posters advertising FH self-screening were placed in each practice’s waiting room and nurses’ rooms. These posters contained a QR code that directed patients to the survey. Second, patients who had consented to text message communications with their practice and had a face-to-face GP appointment were sent a text message prompt approximately 48 hours prior to their appointment. This message contained an embedded web link to the survey.
A participation information sheet and consent page were provided when the link was opened. Participants were given the option to provide their contact details and consent for the study team to contact them for a follow-up interview about the screening procedure.
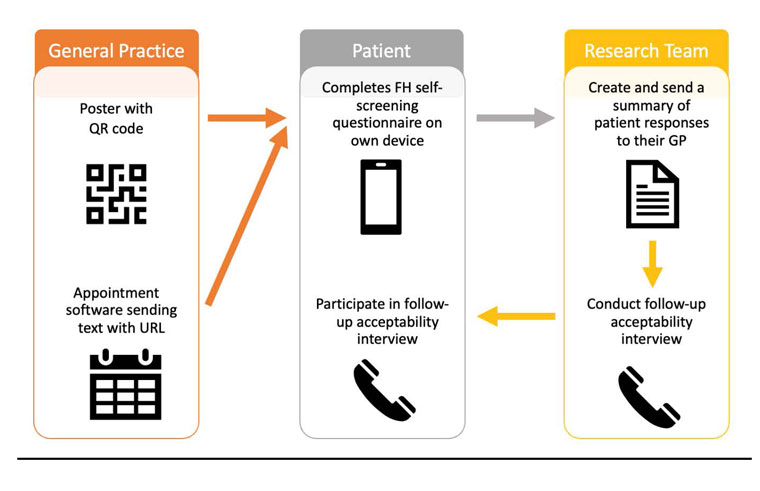
Figure 1. Flow of the familial hypercholesterolaemia (FH) self-screening study.
GP, general practitioner.
FH self-screening questionnaire
The Dutch Lipid Clinic Network Score (DLCNS) is a validated set of criteria used to determine FH risk. It comprises items covering personal and family history of CVD, as well as personal and family history of the physical stigmata of hyperlipidaemia.13 The DLCNS items were adapted to a patient questionnaire format in this study. The items also included images to illustrate some of the physical signs of hyperlipidaemia (eg tendon xanthomata and arcus cornealis).
Integration of patients’ screening results into eMRs
On completion of the questionnaire, participants had the opportunity to provide their email address and instantly receive a copy of their responses. Those who provided their details had a summary emailed to their practice. These summaries were then uploaded into their GP’s correspondence inbox. Participating GPs were encouraged to review the summary of the FH screening and discuss this with the patient. The management pathway was at the discretion of the treating GP; it was not within the scope of this study to examine further investigations or management that occurred after screening.
Acceptability interview
A short, structured interview guide was developed that explored participants’ experiences and acceptability of the self-screening tool. The interview questions were based on the theoretical framework of acceptability and guided by previous healthcare acceptability studies.14–17
Participants who consented to follow-up were contacted by telephone. Three researchers conducted the interviews (SE, DJ and LJ), and the interview procedure was standardised across interviewers. Participants were asked to rank the usability of the self-screening tool on a scale from 1 to 5 (1=very difficult; 2=difficult; 3=neutral; 4=easy; 5=very easy). Interviews were voice recorded with patient consent, omitting any identifying details, and were then transcribed verbatim.
Analyses
Basic descriptive statistical analyses (frequencies, percentages, means) were performed on the quantitative results of the self-screening questionnaire using Microsoft Excel. Patient scores were stratified into risk categories based on the DLCNS diagnostic criteria as follows: >3 low risk; 3–5 intermediate risk; 6–8 high risk; and ≥8 very high risk.
Transcripts were stored and coded in NVivo 12, and data analysed using an iterative thematic analysis approach.18 The analyses used inductive (data-driven) and deductive (research-driven) approaches. SE and ED coded each transcript based on the identification of similar concepts, ideas and patterns in the data and with reference to the key evaluation questions. Once an initial set of codes was derived for each interview question, SE, DJ and LJ grouped these into themes. Rigor was addressed by an iterative process of constant comparison to code and analyse is of the data (moving between codes/emerging themes and transcripts) and continual discussion of emerging themes within the team.
Access to questionnaire and responses
More information on the study’s questionnaire, network score and acceptability interview questions are available from the corresponding author on request.
Results
Practice and patient participation
Four practices in the Greater Sydney area participated. Compared with the general Australian population, these practices were in areas with a greater concentration of older, Australian-born and socioeconomically advantaged residents (Table 1).
In all, 1258 text messages were sent to patients. Of the 234 patients who interacted with the FH self-screening questionnaire, 204 (87%) were recruited via the text notification (response rate 16.2%). Of these, 129 provided their demographic information; 93 (72%) were female and the mean age was 45 years (range 20–79 years).
| Table 1. Characteristics of participating practices and their local government areas |
| |
Practice number |
Australian population |
| 1 |
2 |
3 |
4 |
| Local government area characteristicsA |
| Total population (n) |
78,121 |
182,818 |
213,845 |
230,211 |
25,422,789 |
| Median age (years) |
45 |
38 |
42 |
41 |
38 |
| No. Australian born (% of total) |
61,895 (79.2) |
114,365 (62.6) |
182,826 (85.5) |
179,359 (77.9) |
17,019,815 (66.9) |
| No. Aboriginal or Torres Strait Islander (% of total) |
2101 (2.7) |
2162 (1.2) |
11,759 (5.5) |
3273 (1.4) |
812,728 (3.2) |
| Median weekly household income ($) |
1756 |
2340 |
1623 |
2288 |
1746 |
| IRSADB (decile) |
9 |
10 |
7 |
10 |
– |
| RRMAC classification |
1 |
1 |
2 |
1 |
– |
| Practice characteristics |
| Time posters displayed (months) |
5 |
5 |
5 |
4 |
– |
| Total no. responses via poster |
13 |
3 |
4 |
11 |
– |
| Total no. text messages sent |
484 |
161 |
264 |
349 |
– |
| Total no. responses via text |
80 |
26 |
54 |
44 |
– |
A2021 Census data of corresponding local government areas in which practices were located (available from www.abs.gov.au).
BThe Index of Relative Socio-economic Advantage and Disadvantage (IRSAD) summarises information about the economic and social conditions of people and households within an area, including both relative advantage and disadvantage measures. Areas in lower deciles are those with greater disadvantage and a lack of advantage. Higher deciles are indicative of areas with a lower disadvantage and greater advantage.
CThe Rural, Remote and Metropolitan Area (RRMA) classification divides Australia into three zones and seven classes: metropolitan zone (RRMA 1 and 2), rural zone (RRMA 3–5), remote zone (RRMA 6 and 7). |
FH self-screening
Figure 2 details the results of screening. In all, 137 participants (10.8% of those sent the text message, and 58.5% of those interacting with the tool) were eligible and completed the questionnaire, 68 (29.1% of non-completers) started but did not complete the items and 29 (12.4% of non-completers) interacted with the tool but were not eligible. Of those who did not complete the items, 57 (83.8%) exited after viewing the participant information, five withdrew before reaching questions about family/personal history and six withdrew at the questions relating to the physical signs. Nine participants were identified to be high risk for FH, and seven of these patients provided their details (two males and five females, age range 34–57 years).

Figure 2. Flow chart of the results of familial hypercholesterolaemia screening.
Participant acceptability
Of the 84 participants who consented to an interview, 49 were contactable/available to participate. The average time to complete the self-screening was 3.5 minutes, and all (100%; n=49) found this acceptable. Access to the survey via the text message was rated as ‘very easy’ by 95% (n=41), with the remainder indicating it was ‘easy’. All participants who accessed the survey via the QR code on the poster (n=4; 100%) found accessing the survey ‘very easy’. The usability of the survey platform was evaluated as ‘very easy’ by 93.8%, with the remainder rating it as ‘easy’. Four participants required assistance from others to complete the self-screening.
The participant acceptability interviews elicited several themes (Table 2). Overall, the participants found the self-screening process acceptable, raised awareness in their age group and was simple to perform. When asked about barriers to completing the survey, four themes were elicited, as detailed below.
| Table 2. Major themes elicited from the acceptability interview |
| Interview question |
Major themes |
Illustrative quotes (Practice number, responder designationA) |
| Reason for participation |
Wanting to contribute to research |
‘I think research is important’ (Practice 2, 19)
‘I like participating in studies’ (Practice 2, 30) |
| Concerned about my own risk |
‘My nan has angina and coronary artery disease’ (Practice 2, 32)
‘I had these things happen in the family’ (Practice 2, 38)
‘It was about heart disease and I’m 59’ (Practice 1, 46) |
| I like/trust my GP |
‘I’ve had such a long association with the doctors’ surgery that, since it was coming from them, I was happy to’ (Practice 2, 47)
‘I just like my doctor’ (Practice 1, 25) |
| Boredom |
‘I was bored’ (Practice 3, 2; Practice 4, 14; Practice 1, 36)
‘Boredom’ (Practice 1, 19) |
| Discussing with others |
Raising awareness |
‘To raise awareness for them’ (Practice 3, 12)
‘To get the words out there’ (Practice 4, 14)
‘The earlier you find out about these sorts of things the more easily you’re able to manage that risk’ (Practice 3, 7) |
My family might also
be high risk |
‘Because if I can be at high risk then my sisters can be at high risk’ (Practice 2, 32) |
| Would rather not discuss with others |
‘Several of them have enough health issues of their own without worrying about me’ (Practice 4, 7)
‘I’d probably tell my husband not my kids, just so that he knew’ (Practice 4, 7) |
| Others can support me if I’m diagnosed |
‘They would be able to help support me’ (Practice 3, 27)
‘They would be able to help me out’ (Practice 2, 30) |
| Discussing with your health provider |
I expected the doctor to raise the discussion |
‘Only if he raises it’ (Practice 2, 50)
‘I will talk about it if she plans to talk about it’ (Practice 2, 51)
‘I just imaged that if there was any issues … she would let me know’ (Practice 2, 17) |
| I’m low risk so didn’t need to discuss |
‘There was nothing concerning, so no’ (Practice 2, 42)
‘I don’t think I’m at an increased risk’ (Practice 2, 52)
‘I was a low-risk category, so I don’t feel the need to have that conversation’ (Practice 1, 19) |
| I’ve already discussed heart health with my GP |
‘I’ve discussed the high cholesterol with the doctor’ (Practice 1, 31)
‘I have a cardiologist who I’ve seen’ (Practice 1, 42) |
| Barriers to completing the questionnaire |
I don’t know my family history |
‘It’s hard to answer I guess if you don’t know exactly what your family history is’ (Practice 2, 17)
‘I wasn’t too sure about some of the medical history of my parents’ (Practice 2, 47) |
| It was very easy/simple |
‘I thought it was very good. I breezed through it’ (Practice 1, 46)
‘I think it was very easy, I probably would have just closed the survey if it was too long or difficult’ (Practice 3, 24) |
| Would be better if a medical professional was involved |
‘The data is far better for you guys if it was done in the presence of a doctor or nurse’ (Practice 2, 50)
‘Could be better off being supervised by a medical professional’ (Practice 1, 67)
‘I wasn’t sure about my own eyes looking for the different colour on the outside’ (Practice 3, 6) |
The elderly might not
be tech savvy |
‘If you had an elderly patient, I think it’d be quite difficult for them to navigate that’ (Practice 4, 2) |
AResponder designation corresponds to the patient number, with participating patients numbered from 1 to 49.
GP, general practitioner. |
It was very simple/easy
Several commented that they found the self-screening process straightforward, with some stating that the main reason for them participating was because it was simple and easy.
I don’t know my family history
Some participants found the family history items difficult to answer because they were estranged from family, were unable to clarify history with deceased family members or health issues were not openly discussed within their family.
Would be better if a medical professional was involved
A few participants commented self-screening might have been easier if it had been completed in the presence of a medical professional. This comment was mostly made regarding the physical examination questions where participants stated they were unsure if they had tendon xanthomata and arcus cornealis.
The elderly may not be confident with technology
Although not identified as an issue for themselves, a couple of participants were concerned that self-screening might be difficult for older people due to them being less confident with technology.
When asked about why they participated in self-screening, four main themes were identified, as detailed below.
Wanting to contribute to research
Many commented that they enjoy participating in research or view research as important. Some identified that this came from having family members who are part of the scientific or medical community, and others offered this as a way they could contribute to the progression of science.
Concerned about my own risk
Many participants stated that concerns for their health were a motivating factor to undertake screening. For some, this concern stemmed from their medical history, such as elevated cholesterol, whereas for others, this came from awareness of family with heart disease. Some participants identified their age or parental responsibilities as the reason(s) for being concerned about their health.
I like/trust my GP
A number of participants stated they undertook self-screening because it was connected directly to their trusted GP. Some commented that this made the text message recruitment modality more trustworthy.
Boredom
A few participants stated that they took part in the self-screening out of boredom.
Four main themes were identified when participants were asked about discussing the questionnaire and its results with others, as details below.
Raising awareness
Participants recognised that informing others that they completed the questionnaire might raise awareness of the issue and encourage them to assess their risk. Some identified that by informing others, they would be promoting earlier detection and prevention.
Relatives might also be at risk
Many identified that their family might also be at risk, given the condition is hereditary, and this would be a reason to share information.
Would rather not discuss with others
A number of participants said they would rather not discuss the questionnaire or its findings with anyone so they would not worry others. A few mentioned that they would only inform their partner, but not other family members.
Others can support me if I’m diagnosed
Several participants said they would discuss their results with others for support if they were high risk. Some identified needing physical or emotional support with the diagnosis or getting to appointments.
The vast majority of participants (96%) did not discuss their screening result with any health practitioner, with three main themes emerging, as detailed below.
I expected the doctor to raise the discussion
A large proportion expected their GP to initiate discussion about the screening. Many assumed they were not high risk because their GP did not discuss their results.
I’ve already discussed heart health with my doctor
Some participants stated that they had already had discussions with their GP about their cardiovascular risk or were already seeing a cardiologist and, therefore, did not discuss their results with their GP.
I’m low risk so didn’t need to discuss
Several participants felt that they were low risk, and so did not need to discuss the results based on their screening answers.
Discussion
Summary
This is the first known Australian study to examine the acceptability of a digital FH self-screening tool in a general practice setting. FH self-screening led to favourable detection rates, and participants found self-screening acceptable. This proof-of-concept study shows that with further refinements, FH self-screening might be a viable mechanism for identifying previously undetected cases of FH.
FH self-screening
A previous Australian study that used automated data extraction software to assess FH risk found a smaller proportion than the present study (0.79%) to be potentially high risk (1843/232,139).10 Of these, 800 (0.3% of total) were confirmed to be high risk following GP review.10 However, that study used LDL results, which might have excluded many potential high-risk patients who did not have an LDL measure. The higher rate of high-risk patients observed in the present study might also be due to the patient-reported personal and family history items and physical signs of hypercholesterolaemia. These items are generally poorly recorded in the eMR and often not documented in the fixed fields of the record.7,8 However, these items might have been beneficial for identifying potential high-risk FH patients aged <45 years, because many would not have had lipids assessed based on guidelines.
The results of the qualitative study identified several ways in which FH self-screening could be improved. Some participants found questions regarding family history and clinical signs challenging. Further refinements to the current tool and digital interface relating to these questions would be required for upscaling FH screening.
Limitations of FH self-screening
The overall response rate to the self-screening was low, and recruitment via posters displayed in the practices was especially low. The barrier of interacting with a digital survey was a factor that some participants noted, but is less likely to be a barrier for younger patients who are more comfortable with technology. Furthermore, we did not collect data on subsequent tests/diagnoses that were established among participants identified as high risk.
Acceptability survey
The acceptability survey suggested that harnessing patient boredom (eg when patients are in the GP waiting room) might be an opportunistic moment in preventive healthcare, alleviating time constraints during consultations.
Survey participation secondary to trust of one’s GP was another common reason for engagement. This finding supports the notion that strong patient rapport and continuity of care are important in achieving preventative health goals and optimising health outcomes.19–21 However, our data showed low rates of patient-initiated discussion around their screening results with their GP. In the present study, there was a time delay in the screening results being imported into the eMR; therefore, the results might not have been ‘top of mind’ for the GP at the next consultation. Strategies to prompt GPs and patients to discuss the results could be integrated into the self-screening tool, such
as screen pop-ups for GPs and SMS reminders to patients.
Although many participants identified a potential familial implication associated with a high-risk result, some expressed not ‘wanting to worry others’. Given that poor health outcomes associated with FH are highly preventable, this lack of understanding might be a contributing factor to both underdiagnosis and the morbidity associated with FH, and emphasises the importance of patient education.6 It would be of value to further explore the GP perspective on FH self-screening concerning perceived benefits for case identification and cascade screening.
Limitations of the acceptability survey
Conducting the acceptability questionnaire via telephone might have limited participants’ responses because it warranted an immediate response. The desire to please the doctor/researchers conducting the survey, especially as patients identified a like/trust for one’s GP as the reason for participation, might have skewed patient feedback. Furthermore, there was a delay of days (or weeks, in some instances) between completing the screening and participating in the acceptability interview, which might have influenced the accuracy of participants’ feedback.
Implications and future directions
Programs that can provide fully integrated preconsultation questionnaires to patients are currently being used in Australian practices, and adopting these technologies to distribute self-screening surveys enables patients to have responsibility and an active role in their healthcare.20 Further iterations and upscaling of the self-screening model used in this study could be improved by providing self-screening tablets in GP waiting rooms where patients may be able to visualise pictures of clinical signs of hypercholesterolaemia more clearly. Furthermore, a fully integrated self-screening tool that instantly imports screening results into the patient file and prompts the GP when the file is opened might operate more seamlessly into the practice workflow.
We are addressing the low rates of screening and feedback from the process evaluation to develop a more streamlined system of patient self-screening that integrates with practice IT systems and fits more seamlessly in GPs’ workflow. This next iteration will permit the screening results to be instantly available to the GP; data from screening rates and case identification will assess whether these improvements increase the screening rate. These results will then be linked with evidence-based management recommendations. The increasing use of artificial intelligence in health might also be used to identify individuals at increased risk of FH using algorithms that scan data in the free- and fixed-text components of the medical record, so that individualised screening questions are sent to patients.
However, in the interim, GPs can identify cases of FH through data extractions of pathology test results and family histories of their patient database. This can be done in most medical record software, and can be automated to identify cases on a regular basis.
Conclusion
This proof-of-concept study showed that FH self-screening can be implemented in general practice. Further refinements need to be undertaken to improve patient participation. However, the model was acceptable to patients and successfully identified those at high risk of FH. If implemented more broadly, FH self-screening could increase the detection of FH, lowering rates of preventable CVD.