Chronic pelvic pain (CPP), defined as pain perceived to originate from the pelvis lasting more than six months, is a debilitating but common condition affecting 26% of women worldwide.1,2 It also poses a significant economic burden on health systems as CPP accounts for approximately 20% of all gynaecology outpatient appointments and up to 40% of gynaecological laparoscopies.3,4 CPP is a challenging and often multifactorial clinical syndrome, with multiple possible differential diagnoses. In patients with CPP for which no alternate cause can be identified, studies have suggested that pelvic congestion syndrome (PCS) can have a prevalence of 30–40%.5,6 This prevalence might be underestimated due to poor awareness of PCS as an aetiology for CPP, no standardised diagnostic criteria and a lack of recent high-quality research in the area.
PCS consists of a group of clinical symptoms associated with pelvic venous insufficiency – usually reflux of the ovarian or internal iliac veins.6 PCS has a substantial effect on patients, clinicians and health networks. However, it is a disease entity that is still poorly understood, underdiagnosed and, therefore, undertreated, with the average time to diagnosis being reported as up to four years after initial presentation.7
Aim
PCS poses a diagnostic challenge to general practitioners (GPs) and gynaecologists in the differential diagnosis of patients presenting with CPP. This review explores the pathophysiology, symptomatology, investigation and treatment of PCS.
Pathophysiology of PCS
The female pelvic viscera are drained by a rich anastomotic plexus of veins, including ovarian, para-ovarian, uterine, vesicular, rectal and vulvar veins. These channels predominantly rely on vascular tone and gravity for drainage and are relatively valveless.8 In PCS, these vessels, particularly the ovarian veins, are incompetent and enlarged, with stagnation or reflux of blood flow as demonstrated schematically in Figure 1. This incompetence results in pelvic venous hypertension and dilated congested pelvic varicosities involving the uterus, rectum, bladder and vagina. The aetiology of these changes is poorly understood but is hypothesised to be secondary to both hormonal and anatomic dysfunction, which are particularly exaggerated during pregnancy.9 The vasodilatory effects of oestrogen and progesterone are thought to contribute to ovarian venous dilatation and PCS.10 Further, pregnancy is associated with a 60% increase in the capacity of the pelvic veins and venous kinking associated with the malpositioned gravid uterus. These changes are thought to persist upon the completion of the pregnancy, with the pelvic veins failing to return to normal size and function.8 To support these theories, PCS has been found to most commonly affect women of reproductive age (between 20 and 45 years), with parity being a well-established risk factor.11,12 It is increasingly accepted that it can occur in younger nulliparous women and is associated with congenital venous abnormalities.13 Rarely, pelvic venous hypertension can be associated with extrinsic venous compression such as May-Thurner syndrome (iliac vein compression between the iliac artery and the spine) or nutcracker syndrome (renal vein compression between the aorta and the superior mesenteric artery).14
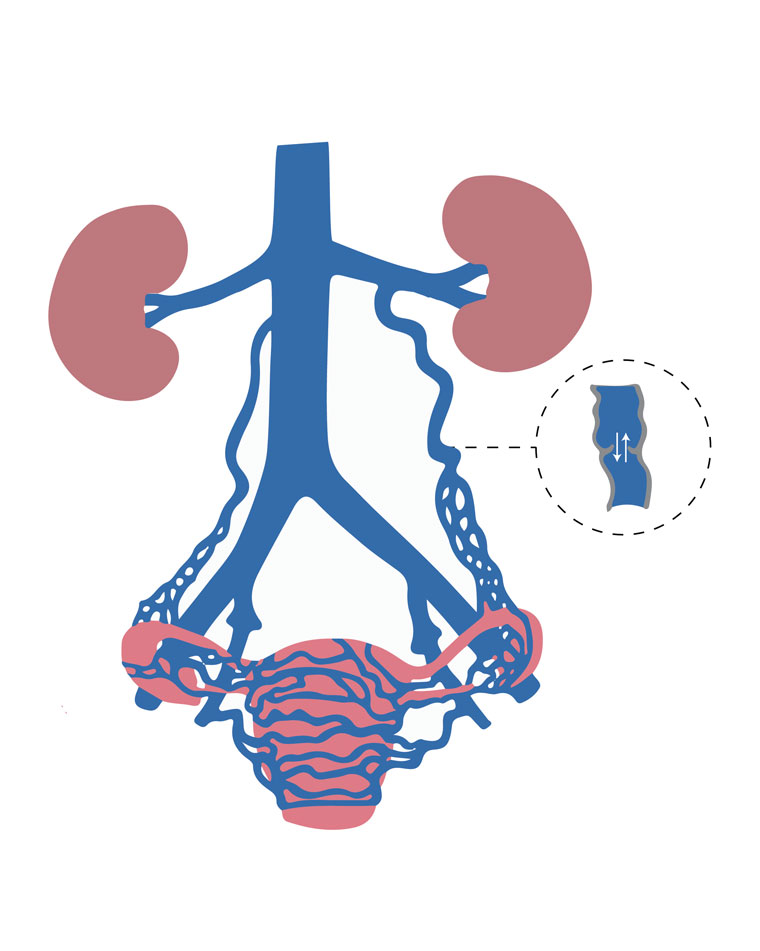
Figure 1. Schematic illustration representing ovarian and internal iliac vein reflux leading to pelvic varicosities.
Adapted from Liang E. Pelvic congestion syndrome. Sydney Fibroid Clinic, 2019. Available at www.sydneyfibroidclinic.com.au/pelvic-congestion/about-pelvic-congestion, with permission from Liang E.
Clinical evaluation
The characteristic pain is perceived to originate from pelvic organs/structures, can be cyclical or non-cyclical, and typically lasts longer than six months. Pelvic pain that is venous in origin is typically felt as a dull ache/heaviness and can be unilateral or bilateral. Symptoms are often worse with walking and prolonged standing and during and after coitus.15,16 Irritable bowel symptoms, bloating, dyspareunia, vulval varicosities and lower limb venous pathologies are commonly associated with PCS. High clinical suspicion should be raised with ongoing pelvic symptoms in patients when other gynaecological pathology has been optimised or ruled out. On clinical examination, patients often have lower abdominal, adnexal tenderness on bimanual examination and might have visible vulvovaginal, gluteal, perineal or lower limb varices. Table 1 outlines possible pathologies to consider when evaluating a patient with CPP.
| Table 1. Differential diagnoses of chronic pelvic pain |
| Gynaecological |
Urologic |
Gastrointestinal |
- Endometriosis
- Leiomyoma
- Adenomyosis
- Ovarian remnant syndrome
- Pelvic inflammatory disease
|
- Interstitial cystitis
- Radiation cystitis
- Bladder cancer
|
- Irritable bowel syndrome
- Inflammatory bowel disease
- Colorectal carcinoma
|
| Musculoskeletal |
Neurological |
Vascular |
- Abdominal wall
- myofascial pain
- Pelvic floor tension myalgia
- Fibromyalgia
- Coccygodynia
|
- Abdominal wall cutaneous nerve entrapment
- Central sensitisation
|
- Pelvic congestion syndrome
|
Investigation
Imaging is a critical part of the work-up of patients with suspected PCS to characterise pelvic venous changes. Ultrasound assessment is considered a first-line investigation as it is non-invasive and inexpensive and does not expose the patient to radiation. Ultrasound can be performed through transabdominal and transvaginal approaches. Transabdominal ultrasound can demonstrate pelvic varicosities and enable accurate examination of the left ovarian vein. The transvaginal approach, however, is considered the examination method of choice, as it enables a more accurate examination of the pelvic venous plexus compared to the transabdominal approach.12 Both techniques can be combined with Doppler imaging and provocation manoeuvres such as Valsalva, tablet tilt or standing examinations to look for retrograde flow or flow reversal suggestive of reflux.17 It is important to note that Doppler examination of the ovarian and pelvic veins is not routinely included in a standard pelvic ultrasound and should be specifically requested by clinicians who suspect a diagnosis of PCS.
Computed tomography (CT) and magnetic resonance venography (MRV) are also widely used in the work-up of CPP and can show vein diameter and the presence of pelvic varices. Both modalities can also provide a detailed anatomical overview and rule out other pathologies. A 2018 systematic review by Steenbeek et al suggested that MRV was as effective as ultrasonography in diagnosing PCS. However, due to only a small number of heterogenous studies available in the literature, they were unable to draw a firm conclusion.18 A small retrospective study showed that CT and MRV were equivalent; however, there is no high-quality evidence to support this finding.19 Both CT and MRV are also performed in the supine position, which might result in underdiagnosis of PCS in the early phases, as there is less venous engorgement compared to ultrasonography, which can be performed in dynamic positions.18 Another consideration is that CT imaging requires radiation and should generally be avoided in this cohort of patients who are often premenopausal.16
Catheter-directed venography of the ovarian and internal iliac veins remains the reference standard for the diagnosis of pelvic venous pathology. It has all the aforementioned benefits of allowing provocation manoeuvres and assessing retrograde flow and demonstrates filling of contralateral veins or reflux in tributaries. However, venography is costly and invasive and is not commonly performed as a
first-line investigation.
Although demonstration of ovarian vein dilation on imaging might suggest PCS as the aetiology driving a patient’s CPP, a 2010 systematic review suggested that these findings can be found in asymptomatic patients or those with an alternate cause for pelvic pain, which only contributes to the diagnostic difficulties.20
Treatment
There is a sparsity of up-to-date, high-quality literature to guide clinicians in the treatment of PCS. The available evidence, however, describes several therapeutic options that have been shown to successfully alleviate pain in patients suffering from PCS, including medical, surgical and endovascular therapies, which are summarised in Table 2.
Medical treatment of PCS can involve symptomatic, hormonal or venoactive therapy. Of note, medroxyprogesterone acetate (MPA) and gonadotropin-releasing hormone (GnRH) agonists such as goserelin have been used to suppress ovarian function and increase venous contraction. However, the effects of these drugs are often short-lived and they are not efficacious in the long term.21
Surgical ligation of the ovarian veins, either through an open retroperitoneal or laparoscopic approach, was also historically performed for primary ovarian vein incompetence with varying results. However, this procedure is performed with the patient supine, with the patient’s abdomen insufflated with pressurised carbon dioxide, which might result in the underestimation of the number of varices and, therefore, decrease procedural efficacy.22 Further, this procedure exposes the patient to a general anaesthetic and a long recovery period and, therefore, is no longer commonly performed.
Ovarian vein embolisation (OVE) is an endovascular procedure that is performed in a catheterisation laboratory or interventional suite and has emerged as the preferred gold standard treatment for PCS. It is performed under local anaesthetic with or without sedation and can be safely performed in an ambulatory vein clinic.23 The procedure involves venous access, usually into the common femoral vein followed by diagnostic venography to characterise and identify insufficient venous axes, which are subsequently embolised and occluded using platinum coils or sclerotherapy. An example of reflux of the right ovarian vein is shown in Figure 2A and coil embolisation in Figure 2B. Patients are discharged on the day of the procedure. Reported complications range from 0.85% to 10% and are usually minor without sequelae. These include access-site haematoma, contrast reaction, coil migration (managed with snaring during the same procedure) and embolisation of a non-target vein (which can usually be retrieved during the same procedure). Although there is a paucity of randomised studies comparing embolisation to placebo, the technical success rate of OVE in large cohort studies has demonstrated a high technical success rate of 98–100%, with symptom improvement at one to five years of follow-up in 80–93% of patients.16,24–27 Furthermore, a 2003 randomised control trial by Chung and Huh demonstrated embolotherapy as significantly more effective at reducing pelvic pain compared to medical therapy and hysterectomy.24
| Table 2. Summary of treatment methods for pelvic congestion syndrome |
| Treatment |
Examples |
| Medical |
| Symptomatic therapy |
- Simple analgeisa
- Gabapentin/amitriptyline
|
| Hormonal therapy |
- Medroxyprogesterone acetate
- Gonadotropin-releasing hormone agonist
|
| Venoactive therapy |
- Micronised purified flavonoid fraction
|
| Surgical |
| |
- Open retroperitoneal ovaerian vein ligation
- Laparoscopic ovarian vein ligation
- Hysterectomy with salpingo-oopherectomy
|
|
Endovascular |
| |
- Ovarian vein embolisation
|
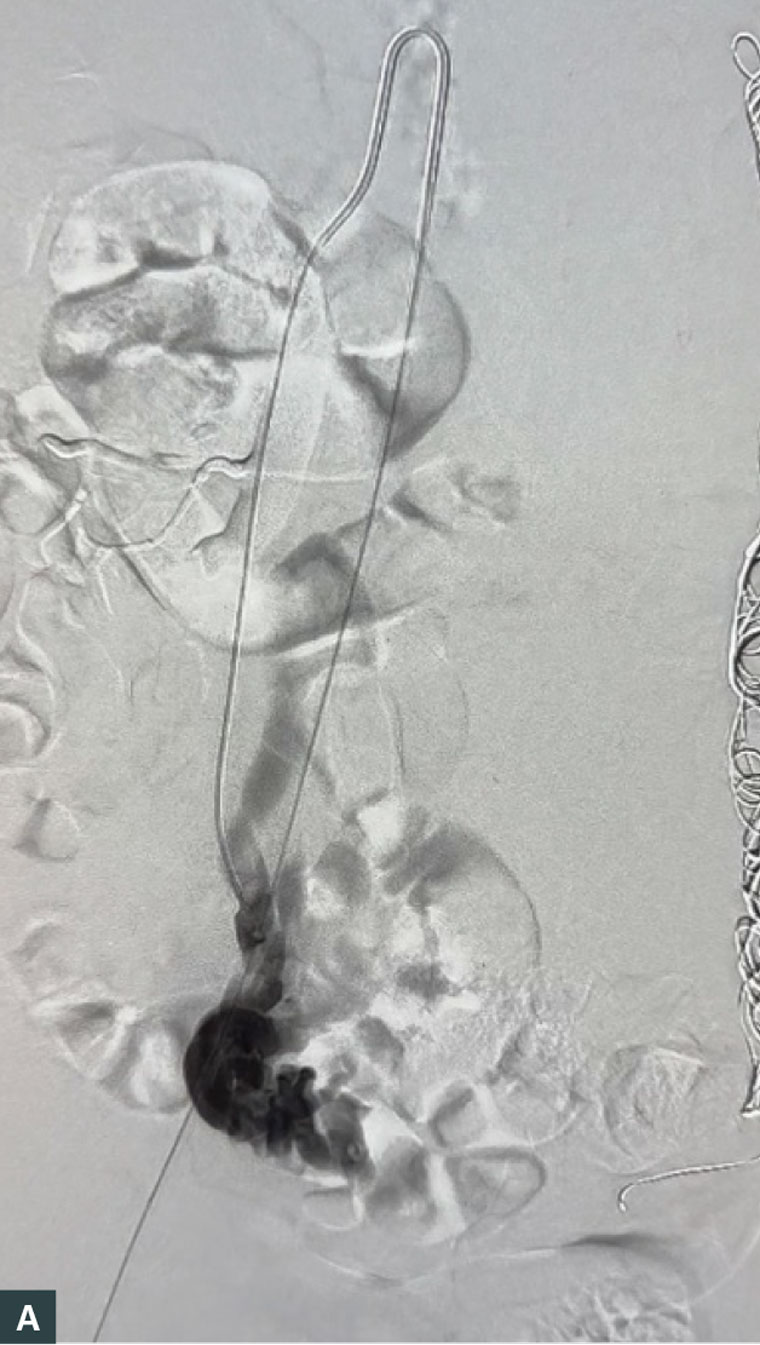
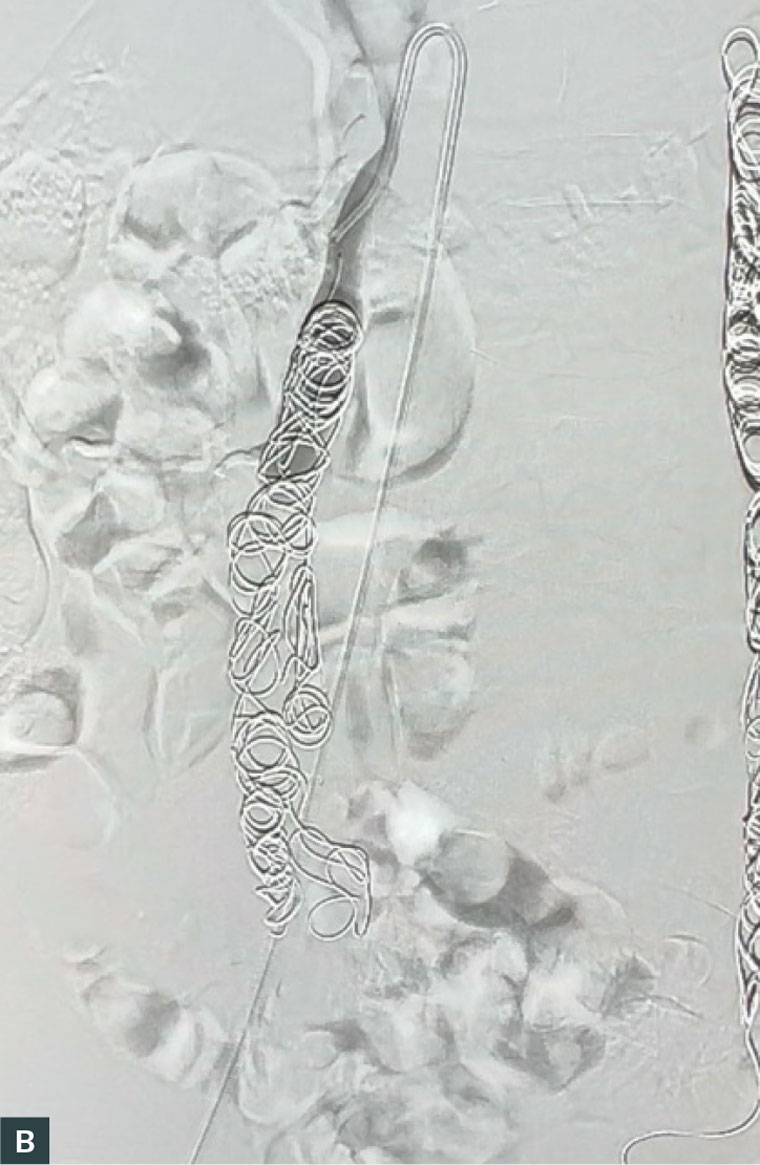
Figure 2. (A) Venography of the right ovarian vein, showing reflux and pooling of contrast in the pelvic veins and (B) venography after coiling of the right ovarian vein, showing occlusion of the vein and no further pooling of contrast.
Conclusion
This clinical summary highlights the key aspects of PCS, a condition often overlooked but associated with significant morbidity in women. PCS manifests as CPP, which can greatly affect a patient’s quality of life. This summary emphasises the importance of considering PCS as a potential diagnosis in women with refractory CPP and highlights the various diagnostic modalities available, including imaging techniques and minimally invasive procedures, which can aid in confirming the presence of pelvic venous insufficiency. Further, this summary provides an overview of the treatment options, ranging from conservative measures to endovascular interventions, which aim to alleviate symptoms and improve patient outcomes. By increasing awareness and understanding of PCS among healthcare professionals, this clinical summary promotes early recognition and appropriate management of this potentially underdiagnosed condition, ultimately improving patient care and enhancing their quality of life.
Key points
- CPP is a common but challenging presentation for GPs and gynaecologists.
- PCS contributes to 30–40% of CPP where no other cause is found but is not considered and therefore might be underdiagnosed.
- PCS can be relatively easily diagnosed with transvaginal ultrasound.
- Ovarian vein embolisation is a safe, minimally invasive and efficacious treatment of PCS.
- Better awareness and clinical suspicion for the symptomatology of PCS might speed up diagnosis and treatment.