Chronic kidney disease (CKD) is a common healthcare problem affecting 1 in 10 adult Australians.1 At the beginning of the 2020s, an estimated 2.5% of working-age Australians were living with more advanced CKD Stage 3–5.1 Over 15,000 people were on dialysis, and more than 13,000 were living with kidney transplants.2 Diabetes was the leading cause of end-stage kidney disease (ESKD), accounting for 40% of cases.2
CKD is an independent risk factor for cardiovascular and all-cause mortality. The adjusted mortality risk increases significantly with disease progression and, compared with the general population, doubles in patients with CKD Stage 3 and is three-fold greater in those with CKD Stage 5.3 CKD costs the Australian government nearly $5 billion annually, with over $1 billion alone needed for ESKD requiring renal replacement therapy.1
It is estimated that the incidence of CKD in Australia will increase, with the number of new patients with CKD Stage 3–5 predicted to exceed 160,000 by 2030, with a two-fold increase in the number needing renal replacement therapy.1
Preventing only 10% of CKD cases from advancing to these late stages could result in almost 550 years of life and over $1.5 billion saved, emphasising the need for early diagnosis and instigating measures to prevent disease progression.1
Renin–angiotensin–aldosterone system (RAAS) blockade has been the mainstay of managing CKD for the past few decades. However, there has been an unmet need for additional treatment options for additive effects to RAAS blockers. In recent years, sodium–glucose cotransporter 2 (SGLT2) inhibitors have emerged as promising agents, with growing evidence for cardiorenal protection. If used appropriately, SGLT2 inhibitors can significantly improve the outcome for CKD patients.
Aim
This article aims to provide evidence-based information to general practitioners, aiding the decision to initiate SGLT2 inhibitors for CKD in their day-to-day practice.
What are SGLT2 inhibitors and how do they protect the kidneys?
SGLT2 receptors are proteins in the proximal tubules responsible for reabsorbing almost 90% of filtered glucose coupled with sodium. The primary effect of SGLT2 inhibitors is to block sodium and glucose reabsorption, leading to glucosuria, natriuresis and osmotic diuresis.4 Increased sodium delivery to the macula densa in the distal tubules stimulates vasomotor changes that reduce the intraglomerular pressure.
This effect preserves renal function and reduces proteinuria in the long term.5 A modest but sustained reduction in blood pressure, weight loss, decreased inflammation and improved cell survival are the additional factors contributing to the renoprotective effects of SGLT2 inhibitors. Furthermore, increased distal sodium delivery promotes urinary potassium excretion, which might facilitate uptitration of RAAS blockage to a more effective dose with less risk of hyperkalaemia.5,6
What is the evidence for the benefits of SGLT2 inhibitors in CKD?
The renoprotective effects of SGLT2 inhibitors were initially reported in cardiovascular outcome trials involving patients with type 2 diabetes (T2D) as secondary outcomes.7–9 Subsequent randomised controlled trials looking at primary renal outcomes provided more convincing evidence of the effectiveness of SGLT2 inhibitors in slowing CKD progression across a broader patient population (Table 1). The results showed that canagliflozin, dapagliflozin and empagliflozin significantly reduced the progression of CKD.10–12 Although canagliflozin was only used for patients with T2D with diabetic kidney disease, dapagliflozin and empagliflozin were shown to be effective regardless of diabetes status, renal diagnosis, CKD stage, estimated glomerular filtration rate (eGFR) at enrolment, race, gender and the presence of proteinuria. However, the proportional risk reduction was more pronounced in patients with a higher urine albumin:creatinine ratio, a group with a higher risk of disease progression.10–12
Is any SGLT2 inhibitor superior to others?
At the time of this review, no head-to-head trials had compared different SGLT2 inhibitors. Evidence from renal outcome studies shows that empagliflozin, dapagliflozin and canagliflozin are all effective in preserving renal function with comparable results and are presumed to have a class effect (Table 1). Considering this, one can extrapolate that physicians can choose the SGLT2 inhibitor that is most suitable for their CKD patients (Figure 1). A prespecified analysis of the Dapagliflozin on Renal Outcomes and Cardiovascular Mortality in Patients with CKD (DAPA-CKD) trial showed that dapagliflozin caused a more significant reduction in CKD progression in T2D patients with a higher HbA1c level and a higher urine albumin:creatinine ratio, and might be a preferred agent in this group of patients.13 Of the three SGLT2 inhibitors (canagliflozin, empagliflozin, and dapagliflozin) used in major trials, Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE), Empagliflozin in Patients with Chronic Kidney Disease (EMPA-KIDNEY), and DAPA-CKD (Table 1), only dapagliflozin and empagliflozin are commercially available in Australia for clinical use. Dapagliflozin and empagliflozin are both subsidised under the Pharmaceutical Benefits Scheme for CKD using DAPA-CKD criteria (Table 1). Patients with polycystic kidney disease, lupus nephritis, antineutrophil cytoplasmic antibody–associated vasculitis, patients with an organ transplant and patients on immunosuppressive therapy for kidney diseases are not eligible.
| Table 1. Summary of renal outcome with sodium–glucose cotransporter 2 inhibitors randomised controlled trials |
| Study |
Summary |
Interventions |
Renal-specific outcomes |
| CREDENCE 201910 |
- Age ≥30 years with T2D and
- DKD eGFR 30–89 mL/min/1.73 m2
- uACR 33.9–565 mg/mmol
- Stable dose level of RAAS blocker
- Patients with non-DKD, T1D, history of renal transplant, dialysis or immunosuppression excluded
|
- Canagliflozin 100 mg daily vs placebo
|
- Median follow-up 2.62 years
- Canagliflozin caused a 34% reduction in the
- relative risk of the renal-specific composite of
- ESKD, a doubling of the creatinine level or death from renal causes
- Canagliflozin caused a 32% reduction in the relative risk of ESKD
- Mean uACR was 31% lower in the canagliflozin group
|
| DAPA-CKD 202011 |
- Patients with diabetes (67.5%) and without diabetes (32.5%)
- eGFR 25–75 mL/min/1.73 m2
- uACR 22.6–565 mg/mmol
- Stable dose level of RAAS blockerA
- Patients with T1D, APKD, ANCA vasculitis, lupus nephritis, history of immunosuppression within 6 months excluded
|
- Dapagliflozin 10 mg daily vs placebo
|
- Median follow-up 2.40 years
- Dapagliflozin was associated with a 39% reduction in the primary outcomes of a sustained decline in eGFR by >50%, ESKD and renal or cardiovascular death
- Mean urine ACR was 29.3% lower in the dapagliflozin group
|
| EMPA-KIDNEY 202212 |
- eGFR 20–44 mL/min/1.73 m2, regardless of albuminuria
- eGFR 45–89 mL/min/1.73 m2 with uACR ≥22.6 mg/mmol
- Patients with diabetes (46.2%) and without diabetes (53.8%)
- Stable dose level of RAAS blockerA
- Patients with APKD and renal transplant were excluded
|
- Empagliflozin 10 mg once daily vs placebo
|
- Median follow-up 2 years
- Compared with placebo, empagliflozin reduced the risk of progressive kidney disease (ESKD, a sustained decrease in eGFR to <10 mL/min/1.73 m2 or a sustained reduction in eGFR of ≥40% from baseline, or death from renal causes) or death from cardiovascular causes by 28%
|
APatients unable to receive renin–angiotensin–aldosterone system (RAAS) blockers due to any reason were eligible for inclusion in the study.
ACR, albumin:creatinine ratio; ANCA, antineutrophil cytoplasmic antibody; APKD, adult polycystic kidney disease; DAPA-CKD, Dapagliflozin on Renal Outcomes and Cardiovascular Mortality in Patients with CKD trial; DKD, diabetic kidney disease; eGFR, estimated glomerular filtration rate; EMPA-KIDNEY, Empagliflozin in Patients with Chronic Kidney Disease trial; ESKD, end-stage kidney disease; T1D, type 1 diabetes; T2D, type 2 diabetes; uACR, urine albumin:creatinine ratio. |
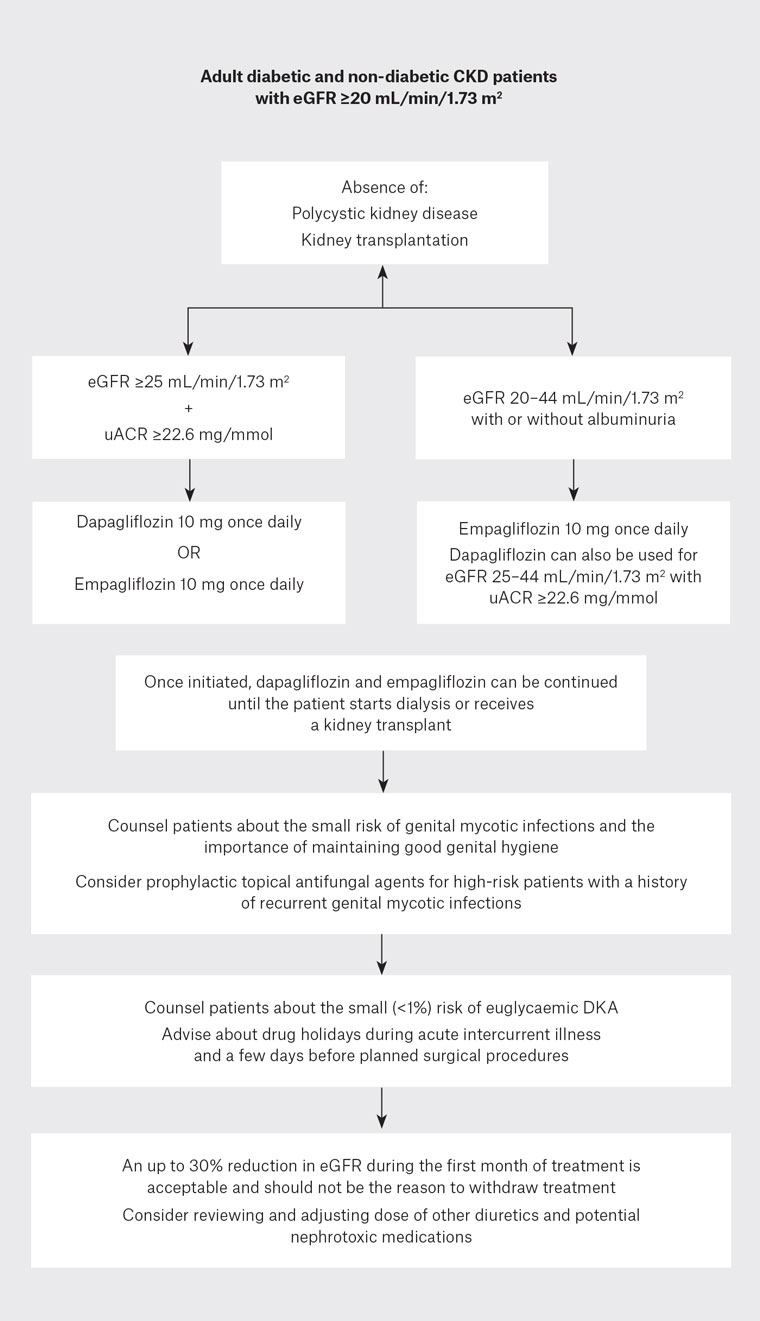
Figure 1. Flow chart for the use of sodium–glucose cotransporter 2 (SGLT2) inhibitors in patients with chronic kidney disease (CKD).
DKA, diabetic ketoacidosis; eGFR, estimated glomerular filtration rate; uACR, urine albumin:creatinine ratio.
What is the risk of serious adverse effects of SGLT2 inhibitors?
The safety of SGLT2 inhibitors was established in large randomised controlled trials and meta-analyses (Table 2).10–15 Apart from the known increased risk of mycotic genital infections, there was no evidence of an increased risk of serious urinary tract infections, Fournier’s gangrene, lower limb amputations and fractures.10–15 Studies have not shown an increased risk of severe volume depletion and dehydration.11,12 A meta-analysis showed that SGLT2 inhibitors reduced the risk of acute kidney injury by 23% in patients with and without diabetes.15
A previous history of mycotic genital infections is not a contraindication for SGLT2 inhibitors. However, patients should be counselled about this possible side effect and advised to maintain good genital hygiene to minimise the risk. Topical prophylactic antifungal agents can be considered in high-risk individuals. Uncomplicated mycotic genital infections can be treated with oral antifungal agents, and discontinuation of SGLT2 inhibitors is usually not needed.16
| Table 2. Risk of possible complications with sodium–glucose cotransporter 2 inhibitors10–15 |
| Complication |
Evidence of increased risk |
| Mycotic genital infections |
Yes |
| Euglycaemic diabetic ketoacidosis |
Yes (<1%) |
| Serious urinary tract infections |
No |
| Fournier’s gangrene |
No |
| Lower limb amputations |
No |
| Fractures |
No |
| Volume depletion |
No |
| Acute kidney injury |
No |
Is there a right or wrong patient to initiate SGLT2 inhibitors for CKD?
SGLT2 inhibitors are effective in slowing CKD progression across a wide range of eGFR (20–89 mL/min/1.73 m2) and renal diagnoses and can be used in most CKD patients.10–12 However, all major trials to date have excluded patients with adult polycystic kidney disease or a kidney transplant, and existing evidence does not support the use of SGLT2 inhibitors in these patient groups.10–12 Similarly, no evidence supports the initiation of SGLT2 inhibitors in patients with eGFR below 20 mL/min/1.73 m2. However, if already on an SGLT2 inhibitor, patients can continue treatment until they reach ESKD or are transplanted.10–12
Although 2.2% of patients in the EMPA-KIDNEY trial had type 1 diabetes (T1D), other trials excluded patients with T1D, and there are limited data on the use of SGLT2 inhibitors in T1D to make a firm recommendation.12,14
SGLT2 inhibitors are equally effective in slowing CKD progression regardless of the eGFR at the initiation of treatment; however, considering the high morbidity and mortality associated with more advanced stages of CKD, initiating SGLT2 inhibitors at an early stage would be more beneficial.
Risk of euglycaemic diabetic ketoacidosis with SGLT2 inhibitors
There have been concerns about euglycaemic diabetic ketoacidosis (DKA) with SGLT2 inhibitors. However, the risk remains minimal (<1%), primarily limited to patients with diabetes.10–12,17 Intercurrent illness, surgical stress, trauma, alcohol misuse, female gender, lean body mass, longstanding T2D and a more than 20% reduction in insulin dose are possible risk factors for DKA.18 Despite the relatively low risk, patients should be counselled about the possibility of DKA at the initiation of SGLT2 inhibitors and educated about discontinuing the medication during intercurrent illness and two to three days before surgery. Special care should be taken in patients with T1D, and insulin doses should not be reduced by more than 20%.
Is an initial acute drop in eGFR with SGLT2 inhibitors problematic?
Like RAAS blockers, SGLT2 inhibitors cause an acute 10–30% drop in eGFR in the initial two to four weeks of therapy, followed by a sustained, slower, long-term reduction in CKD progression.10–12 Rather than acute kidney injury, this drop represents the fall in intraglomerular pressure, a marker of the effectiveness of therapy and the basis of the long-term renoprotective effect of SGLT2 inhibitors. Studies have shown that patients with a >10% early eGFR decline with SGLT2 inhibitors had better long-term renal outcomes than those with a <10% initial eGFR decline.19,20 Therefore, while remaining vigilant, physicians should anticipate this possibility following starting patients on SGLT2 inhibitors; an up to 30% reduction in eGFR in the initial month of therapy is acceptable and should not be the reason to discontinue treatment.21
Conclusion
SGLT2 inhibitors are new treatment options for slowing CKD progression with good safety data. Treatment is effective in patients with or without diabetes, regardless of the presence of significant proteinuria. However, the benefit might be more pronounced in patients with heavier proteinuria. Along with RAAS inhibitors, SGLT2 inhibitors can potentially transform the landscape of CKD management and should be considered in most CKD patients down to an eGFR of 20 mL/min/1.73 m2.
Key points
- SGLT2 inhibitors significantly slow CKD progression regardless of diabetes status and eGFR at initiation.
- Dapagliflozin can be initiated in patients down to an eGFR of 25 mL/min/1.73 m2 and empagliflozin down to an eGFR of
20 mL/min/1.73 m2.
- An up to 30% acute dip in eGFR is expected in the first month of treatment and should not be the reason to stop treatment.
- Patients should be counselled about the small but possible risks of mycotic genital infections and the importance of genital hygiene.
- Beware of the small risk of euglycemic DKA and counsel patients about drug holidays during intercurrent acute illness.