Case
A woman, aged 72 years, approached our clinic with symptoms of abdominal discomfort and difficulty in micturition and defecation. She had a medical history of hypertension for 10 years and newly onset hyperthyroidism (for three months). She had undergone an ovary cystectomy 25 years ago and a subtotal thyroidectomy operation 15 years ago. She had been postmenopausal for 22 years. On pelvic examination, a mobile, palpable mass from the symphysis pubis to the umbilicus was noted. Transvaginal ultrasonography revealed a cystic mass larger than 15 cm in diameter and ascites. Ultrasound findings of the mass were well-vascularised solid components with smooth contours that resembled a malignant mass. The patient underwent thorax computed tomography and abdominal magnetic resonance imaging, which revealed pleural effusion and the heterogeneous complexity of the adnexal mass (Figure 1). CA-125 was 2289 U/mL (normal range <35 U/mL) and CA 19-9 was 138 U/mL (<37 U/mL), respectively. Further, because the patient had tachycardia, blood thyroid stimulating hormone (TSH), T3 and T4 levels were examined, and hyperthyroidism was determined as present. The blood TSH level was 0.05 uIU/mL (normal range 0.4–3.8 uIU/mL), and T3 and T4 levels were 3.1 ng/mL and 24.6 ng/mL, respectively (normal range for T3 is 0.9–1.95 ng/mL and for T4 is 4.4–12.5 ng/mL).
The patient underwent a laparotomy. At the exploration, there were 2000 cc ascites present in the pelvic area. At the right adnexa, there was a 20×25 cm multilobulated cystic mass (Figure 2). Intraoperative frozen section analyses suggested a mucinous cyst. Total hysterectomy, bilateral salpingo-oophorectomy and omentectomy were performed. The patient tolerated the surgery well. Following surgery, blood plasma thyroid levels regressed to normal levels and tumour markers became negative. Further, ascites and pleural effusion were regressed.
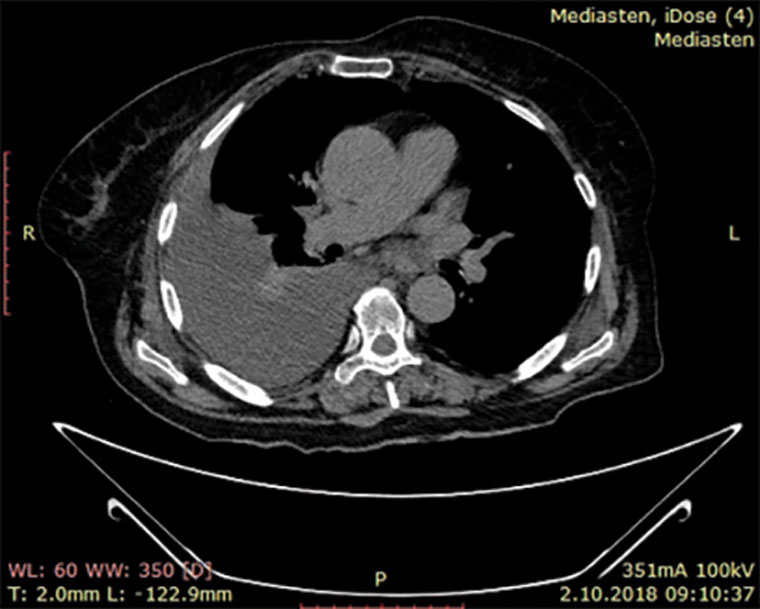
Figure 1. Magnetic resonance image revealing pleural effusion and heterogeneous complexity of adnexal mass.
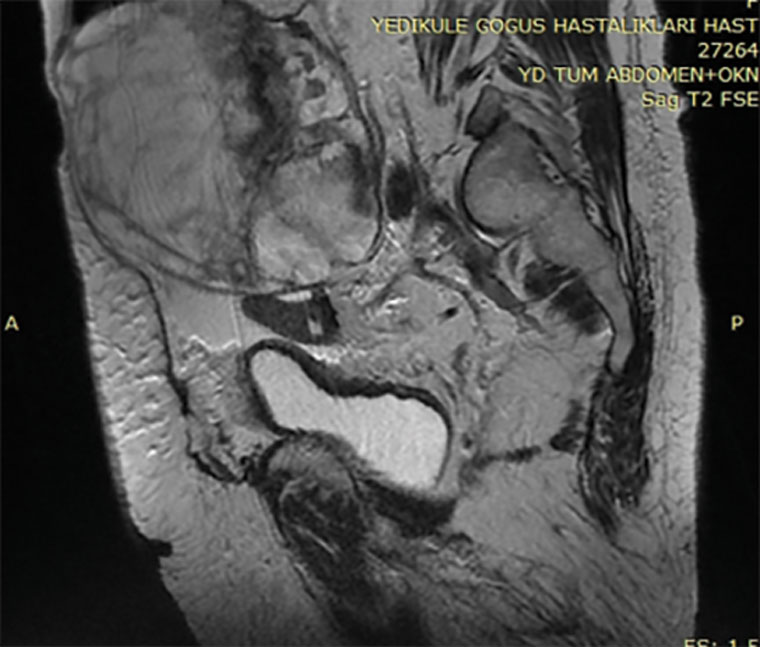
Figure 2. A 20×25 cm multilobulated cystic mass at the right adnexa.
Postoperative histopathological evaluation showed a classic pattern of variable-sized follicles filled with colloid, mature thyroid tissue, ovarian stroma with fibrosis, oedema and corpus albicans (Figure 3). A diagnosis of benign, hormonally active struma ovarii was made. The diagnosis of struma ovarii in this case was based on histopathological criteria of entopic thyroid tissue. Before the surgery was performed, due to hyperthyroidism that had started, the patient had to use methimazole 15 mg daily and propranolol 2×20 mg daily. Still, the blood TSH levels were lower than the normal range. After the operation was performed, there was no need for use of methimazole, and blood TSH, T3 and T4 levels were in the normal range. At six-month follow-up after the operation, the patient’s thyroid hormones were in the normal range. Abdominal ultrasonography was also performed, and no problems were present.
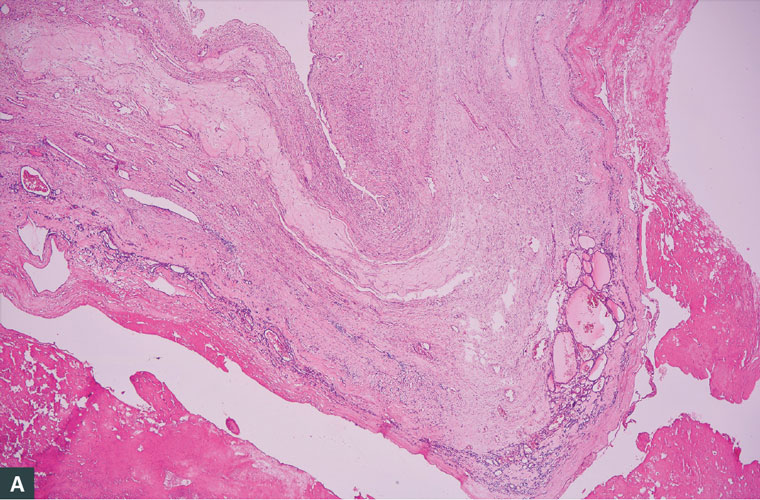
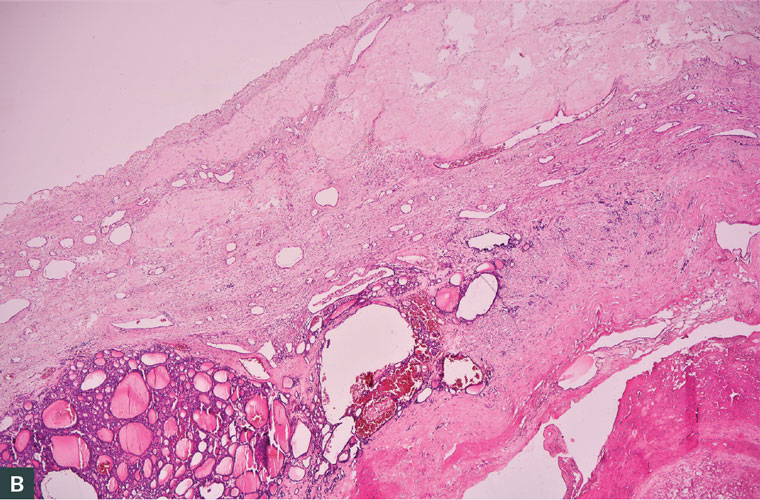
Figure 3. Classic pattern of variable-sized follicles filled with colloid, mature thyroid tissue, ovarian stroma with fibrosis, oedema and corpus albicans. (A) Variable-sized follicles filled with colloid. (B) Ovarian stroma with fibrosis, oedema and corpus albicans.
Question 1
Considering the possible mechanisms of thyrotoxicosis in this woman diagnosed with struma ovarii, what do the thyroid results mean?
Question 2
What are the potential risks for malignancy?
Question 3
What is the aetiology of ascites and pleural effusion?
Answer 1
Thyroid tissue is observed as a component of benign cystic teratomas in 5–7% of cases.1
Thyrotoxicosis occurs in approximately 8% of affected patients. In patients with thyrotoxicosis, the level of TSH is suppressed and the levels of T3 and T4 are elevated. Thyroglobulin is secreted by benign and malignant ovarian stroma, mostly from malignant stroma. Thyrotoxicosis with a benign struma ovarii is rarer, and these women should be treated with antithyroid drugs and beta blockers if needed before the surgery. If the struma ovarii is malignant, the patient must also undergo a thyroidectomy after iodine treatment.
In this report, a rare form of a benign adnexal mass with malignant features was discussed. These hormonally active benign struma ovarii cases are rare. Hyperthyroidism due to struma ovarii was present, which disappeared after the operation. Because of the rarity of these hormonally active tumours, the diagnosis is difficult to make and is mostly done by histopathological evaluation after the operation. Hormonal tumour activity disappears postoperatively and thyroid levels become normalised right after the operation.
Answer 2
Struma ovarii might be benign or malignant. The distinction between these lesions is challenging because there is a lack of firm criteria for diagnosis. Most studies show that the diagnosis could be made based on the presence of entopic thyroid carcinoma (overlapping nuclei, mitotic activity and vascular invasion).1–5 In our case, there was no mitotic activity or vascular invasion. However, entopic thyroid tissue with colloid-filled variable-sized follicles was observed.
Answer 3
The aetiology of ascites and pleural effusion in patients with struma ovarii remains unclear. Meigs described Meigs syndrome in 1937 as cases with benign ovarian tumours with ascites and hydrothorax; the tumours are limited to fibroma, thecoma and granulosa cell tumours. Pseudo-Meigs syndrome consists of ascites and pleural effusion with serous and serosanguinous fluid and tumours such as benign ovarian cysts, uterine leiomyomas and teratomas, including struma ovarii.6 The mechanism of the rise in CA 125 levels in pseudo-Meigs syndrome remains unclear, although Mui et al suggested that the increase in CA 125 levels could be due to the irritation from free fluid leading to the inflammation of pleural and peritoneal surfaces.7 The rise of CA-125 levels and the presence of ascites and pleural effusion in our case are suggestive of a malignant mass. However, the levels of CA-125 normalised on the postoperative eighth day after the cause of the inflammation and irritation was removed. There was no need for any adjuvant therapy after the operation. The thyroid hormone profile of the patient was also normalised after the operation. That is an unusual and rare situation that is noteworthy and a new clinical insight.
Obeidat et al presented 26 struma ovarii cases that mimicked malignant adnexal masses with ascites and raised blood CA-125 levels.8 Preoperatively, all cases were thought to be malignant before the histopathological examination. Similarly, in the present case, the preoperative diagnosis was a malignant adnexal mass. In all cases, blood CA-125 levels normalised and ascites disappeared after the operation. Most of the cases occurred in the fifth decade of life or later. The tumours were between 4 cm and 23 cm in dimension. In the presenting case, the tumour was sized 20×15 cm. The most common presenting symptom was abdominal discomfort, which was the main symptom in the presenting case. In 15 of the cases, pleural effusion was also present. There was no relation between pleural effusion and ascites. Their levels were not affected by each other. Most of the cases were treated with total abdominal hysterectomy and bilateral salpingo-ophorectomy. Just three of the cases studied by Obeidat et al underwent conservative surgery.8 Choosing conservative surgery is related to the fertility wishes of the patient.
Key points
- Thyrotoxicosis with a benign struma ovarii is rarer and should be treated with antithyroid drugs and beta blockers if needed before the surgery.
- If the struma ovarii is malignant, the patient must also undergo a thyroidectomy after iodine treatment.
- Studies show that the diagnosis of malign struma ovarii could be made based on the presence of entopic thyroid carcinoma.