Patients present with symptoms rather than diseases in clinical practice. However, in practice, most pathways and decision support tools focus on diseases as entry points. Breathlessness is a clinically prevalent symptom reported by approximately 10% of adults in the community1 and is associated with a broad range of common conditions such as asthma, chronic obstructive pulmonary disease (COPD), heart failure, ischaemic heart disease, deconditioning, anxiety and obesity. It is also associated with less common but treatable conditions such as pulmonary thromboembolic disease, pulmonary hypertension, interstitial lung disease and valvular heart disease.
The multidimensional aspect and myriad possible causes of chronic breathlessness, ranging from respiratory to cardiac to metabolic diseases, mental health and deconditioning, create a major diagnostic challenge for this very common problem, especially in primary care where most patients first present.2
In the primary care setting, a study of patients presenting with breathlessness reported that less than 30% had a final diagnosis that was fully concordant with their referral diagnosis.3 This finding was supported in another study, where less than 40% of breathlessness patients referred to secondary care with heart failure were confirmed to suffer from heart failure.4 Higher accuracy was reported in a study by Pratter et al in the USA, where 55% of physicians’ predictions following history and physical examination were concordant with the final diagnosis.5 A qualitative study that we conducted revealed that patients and carers experienced misdiagnosis and knowledge gaps of health professionals.6 Those findings aligned with the results of another study we conducted eliciting the perspectives of general practitioners (GPs), multidisciplinary non-GP specialists and allied health professionals.7 That study also identified knowledge gaps in diagnostic testing and constraints on access to these tests as a barrier to optimal care for patients presenting with breathlessness.7
Previously, we published a proposed stepwise approach for the assessment of chronic breathlessness based on a review of diagnostic capacity of priorly evaluated diagnostic pathways that can ascertain the cause in up to 55% of patients with spirometry, electrocardiography and pulse oximetry as initial diagnostic tests.8 Furthermore, 65–90% of presentations were diagnosed when the above approach was combined with a chest X-ray, pathology tests (full blood count, thyroid function and brain natriuretic peptide [BNP]), chest computed tomography (CT) and echocardiography based on the results of prior studies.8 However, none of the algorithms utilised in the proposed diagnostic clinical algorithm were validated or tested in Australia.
The present study aimed to understand the current general practice diagnostic patterns for patients presenting with chronic breathlessness to evaluate the acceptability and feasibility of implementing our proposed diagnostic clinical algorithm (ie a specific series of tests and their sequence) for chronic breathlessness.
Methods
The study consisted of three parts: (1) a nominal group technique (NGT) consensus GP focus group; (2) a focus group using thematic analysis; and (3) analysis of breathlessness referrals from a de-identified primary care breathlessness database cohort. Ethics approval was obtained from the University of New South Wales (UNSW) Human Research Ethics Committee (ID HC200534 and HC200847). Approval was also obtained from the NPS Data Governance Committee and the Australian Department of Health as custodians of the MedicineInsight dataset (2020-032).
Nominal group technique consensus GP focus group
The NGT was conducted by adapting methods from prior studies to fit a virtual design.9 Nine GPs across two meetings participated in this process virtually from among 65 GPs (13.8%) who were invited to participate. They were recruited from a list of GPs who had prior engagements with The George Institute for Global Health, UNSW’s Department of General Practice and the Western Sydney Primary Health Network (WentWest).
Prior to the focus group, practising GPs were provided with a list of 40 diagnostic tests and possible specialty referrals (the list is included in Appendix 1) relating to chronic breathlessness for their consideration based on our prior literature review.8 The focus group was conducted virtually, and participants were asked, in a round robin style, to provide feedback on the list and whether any additional items should be added, based on their experience. New items from the prior focus groups were added to the list of proceeding items. Once no new items were suggested, private voting was completed via an online platform (Qualtrics XM) by ranking the diagnostic tests in response to the prompt ‘What diagnostic tests would GPs be confident in ordering and interpreting to aid in their assessment of breathlessness?’, with a maximum of 35 tests from the whole set. The diagnostic tests were presented in random order to reduce bias. The online platform auto-tallied the responses to generate the median ranks. These were then discussed, and if concerns were raised about the rank, a final vote was conducted to gain consensus. The results of the ranking process were presented descriptively with measures of inter-rater agreement (kappa, Gwet’s AC and percentage agreement). Data analysis was conducted in Stata version 18 (StataCorp).
Qualitative focus group
A convenience sample of practising GPs were recruited from The George Institute, UNSW and health professional public databases using an email invitation. Participants were also asked to suggest peers who might be suitable for the study (snowball recruitment). A semi-structured guide was used for the virtual focus group, where participants were shown the proposed diagnostic clinical algorithm and asked to provide feedback. Focus groups were moderated by AS (male medical graduate, a PhD candidate with prior experience in conducting qualitative studies). Participants were prompted with the question, ‘What do you think are the strengths and weaknesses of the pathway proposed?’ Beyond our proposed pathway, which was developed based on a prior review of past diagnostic pathways,8 participants were also presented with two other pathways commonly cited in the literature (Karnani et al [USA]10 and Berliner et al [Germany]11) developed outside Australia for comparison and contrast. The focus group discussions were recorded and transcribed verbatim. Data were then analysed using thematic analysis using the software NVivo (QSR International) following an approach described by Terry et al.12 Early coding was performed by AS based on the transcript and field notes. Themes developed were then discussed with the other investigators (CJ, AM, CA and GM) to obtain consensus. Further details regarding the qualitative study can be found in our prior study,7 which focused on the participants’ current experience in assessing and managing chronic breathlessness and the support needed.
Primary care routinely collected data analysis
This study utilised NPS MedicineWise’s MedicineInsight primary care dataset of longitudinally collected de-identified electronic health records (EHRs) of 1,961,264 patients from 405 general practice sites in Australia between January 2014 and December 2019.13 The geographic distribution of practice sites was 53.4% from major cities, 23.7% inner regional and 19.9% outer regional. Approximately one-third of practices were from NSW (34.6%), 22.0% were from Victoria, 20.4% from Queensland and 10.2% from Western Australia. Practices were also based in a range of socioeconomic strata, with 14.5% in Socio-Economic Indexes for Areas (SEIFA) Quintile 1 (most disadvantaged) and 21.9% in SEIFA Quintile 5 (least disadvantaged).14 In this dataset, a patient is determined to have presented with breathlessness if the term ‘breathlessness’ or synonyms (eg exertional dyspnoea, shortness of breath and breathing difficulty) were reported as a coded condition or free-text entry in one or more of the Diagnosis, Reason for visit or Reason for prescription fields. Referrals for diagnostic tests recorded in the EHR at any stage of a breathlessness patient’s journey (including those recorded one or two days after the breathlessness presentation) were analysed from free text (further details are provided in Appendix 2). Furthermore, we aimed to identify the main diagnoses in patients presenting with breathlessness and assess whether the tests proposed were feasible and appropriate to include or exclude the diagnoses. Data analysis was conducted in SAS version 9.4 (SAS Institute Inc) and Stata version 18.
Results
Nominal group technique consensus GP focus group
Nine GPs across two focus groups took part in the NGT study. The GPs had overall moderate agreement in their rankings (kappa 0.56 [95% confidence interval [CI] 0.42–0.70], Gwet’s AC 0.60 [95% CI 0.48–0.72], percentage agreement 85.2%).
All the tests in our proposed algorithm except for echocardiography were ranked in the top 10 diagnostic tests employed by most GPs for patients presenting with chronic breathlessness (Table 1).8 GPs preferred starting with pathology tests then proceeding with imaging, before more functional tests such as spirometry. Among the list of diagnostic tests provided, we included an option relating to ‘Response to disease specific therapy’ and referral to a variety of specialties (cardiology, respiratory, psychiatry, geriatrics and exercise physiology). For response to therapy, the median rank was 18 (interquartile range [IQR] 15, 35, percentage agreement 86.1%). Among the specialty referrals, the highest median rank was for a cardiology referral, followed by respiratory, although with a wide IQR (Table 1).
| Table 1. GPs’ diagnostic tests and specialty referral ranking |
| |
n (N=9)
|
Level in proposed algorithm (1–4)A |
Median rank in the GP NGT study (IQR) |
Percentage agreement (ranked in the same tertile)B |
| Diagnostic tests |
| Serum haemoglobin |
8 |
2 |
2 (2, 5.25) |
93.8 |
| Full blood count |
9 |
2 |
3 (1, 4) |
94.4 |
| Basic chemistries |
9 |
NA |
3 (2, 4) |
100 |
| Thyroid function test |
9 |
2 |
4 (2, 6) |
94.4 |
| Chest X-ray |
9 |
2 |
6 (5, 7) |
100 |
| Chest CT scan |
9 |
3 |
6 (5, 8) |
94.4 |
| Electrocardiogram |
9 |
1 |
8 (5, 9) |
75.0 |
| Oxygen saturation |
9 |
1 |
8 (5, 10) |
94.4 |
| Spirometry |
9 |
1 |
9 (7, 12) |
87.5 |
| BNP |
9 |
2 |
13 (9, 17) |
87.5 |
| Ventilation/perfusion scan |
9 |
NA |
13 (12, 20) |
75.0 |
| Lung volumes |
9 |
NA |
13 (13, 28) |
77.8 |
| Arterial blood gas |
8 |
NA |
17 (7.5, 20.75) |
65.2 |
| Flow volume loop |
9 |
NA |
18 (11, 27) |
77.8 |
| EchocardiographyC |
9 |
3 |
19 (11, 21) |
69.4 |
| Specialty referral (assuming they are a diagnostic option) |
| Cardiology |
8 |
NA |
15 (14, 28) |
75.0 |
| RespiratoryD |
3 |
NA |
24 (19.5, 29) |
83.3 |
| GeriatricianD |
3 |
NA |
27 (26.5, 31) |
100 |
| Exercise physiologyD |
3 |
NA |
29 (22.5, 31.5) |
83.3 |
| Psychiatry |
8 |
NA |
33.5 (25, 35) |
89.3 |
ALevel 4 meant a referral to secondary/tertiary care.
BRanks were divided into three groups for this analysis: 1 to 10, 11 to 20 and 20+ (calculated from among those who rank the test/referral).
COnly in the top 15 for one-third of participating GPs.
DOnly available as an option to 4 GPs in the second focus group.
BNP, brain natriuretic peptide; CT, computed tomography; GP, general practitioner; IQR, interquartile range; NA, not applicable; NGT, nominal group technique. |
Qualitative focus group
Eighteen GPs (nine from the NGT) participated across three focus groups (one from South Australia and the remainder from New South Wales, with medical experience ranging from one to 45 years). Qualitative analysis of the focus group discussions elucidated several themes: similarity to current practice; expansion to include clinical observations; and breaking down silos and access to diagnostic tests.
Similarity with current practice
Compared with the two other algorithms presented (Karnani et al [USA]10 and Berliner et al [Germany]11), GPs in the focus group found our proposed algorithm (Figure 1) ‘more aligned with GP practice’ and a ‘more helpful pathway for GPs’ that ‘includes a more holistic review of patients’.8 The GPs felt the other algorithms had less emphasis on ‘psychosomatic causes and were not relevant for GP practice’. The GPs indicated a strong dislike for a prescriptive diagnostic clinical algorithm, especially regarding at which visits to do the test but were more flexible about the test order:
The algorithm should not direct which tests should be done in which visit. (GP4)
Expansion to include clinical observations
Participants identified the need for initial careful history-taking, physical examination and mental health screening being incorporated into the algorithm and had mixed views regarding the utility and access to BNP, lung function testing and echocardiography. GPs wanted ‘greater weight’ assigned to thorough history-taking and physical examination in the algorithm. These add to the knowledge of the patient from the GPs’ long association to provide a holistic assessment of the patient:
BNP is not funded [in practice]. (GP11)
We know the patients well [from a long association], hence can provide a multifactorial assessment for their breathlessness. (GP14)
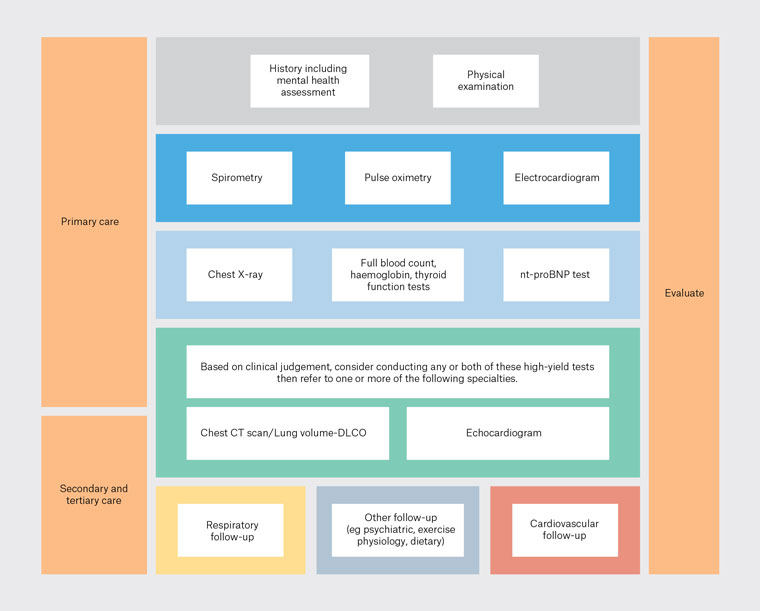
Figure 1. Proposed diagnostic clinical algorithm for chronic breathlessness.
CT, computed tomography; DLCO, diffusing capacity of the lungs for carbon monoxide; nt-proBNP, N-terminal-prohormone brain natriuretic peptide.
Breaking down siloes and access to diagnostic tests in the algorithm
GPs felt that it was important to ensure that chronic breathlessness was not assessed in a ‘siloed manner’ as either cardiac or respiratory but was evaluated holistically. Relating to referrals, GPs raised the usefulness of ‘co-located specialty clinics’, such as a ‘complex breathlessness clinic’ to which they could refer patients who are undiagnosed despite going through the whole diagnostic clinical algorithm rather than to individual specialties, as well as access to secondary care specialists without the need for a referral. They advocated for funding reform to support GP access to diagnostic tests in this breathlessness diagnostic clinical algorithm as some, such as BNP, are currently not reimbursed:
A key thing is to try to have one place to go for those with chronic breathlessness. (GP10)
Something like a metabolic or obesity clinic that’s issue specific rather than needing to refer to gastroenterology, cardiology etc in siloes. (GP12)
How can we evaluate the heart and lungs together? (GP16)
Phoning a consultant [in secondary care] as decision support. (GP2)
Need to consider context, underlying medical conditions and psychosocial setting at the beginning. Chest high-resolution CT scan and echocardiography are easier to arrange than formal lung function testing or DLCO [diffusing capacity of the lungs for carbon monoxide]. (GP14)
Primary care routinely collected data analysis
During the MedicineInsight data collection period (2014–19), 78,912 patients (4.02%) had at least one presentation with breathlessness for a total of 120,218 unique consultations for breathlessness.
All the diagnostic tests in the proposed algorithm are in common use. In this dataset, approximately 45% of patients had a referral for a full blood count followed by approximately 40% for chest X-rays. Considering the prevalence of cardiovascular and airway disease, there was very infrequent use of simple tests such as BNP even when compared to more advanced and expensive tests such as chest CT scans and echocardiography (Table 2).
Twelve diagnoses accounted for over 80% of patient presentations (Table 3). These were diagnoses obtained not only from the consult for breathlessness but included those recorded in follow-up visits up to November 2021. For the most common causes of breathlessness and concurrent diagnoses, the diagnostic tests covered in the pathway would allow GPs to either include or exclude a diagnosis in most cases.
| Table 2. Comparison of diagnostic tests in the proposed pathway as a proportion of patients with breathlessness in the primary care dataset |
| Diagnostic test |
Level in proposed algorithm (1–4)A |
Proportion of patients
n (%) (N=78,912) |
| Serum haemoglobin |
2 |
34,975 (44.3) |
| Full blood count |
2 |
| Chest X-ray |
2 |
29,657 (37.6) |
| Thyroid function test |
2 |
22,473 (28.5) |
| Electrocardiogram |
1 |
16,370 (20.7) |
| Spirometry |
1 |
9260 (11.7) |
| Echocardiography |
3 |
5055 (6.4) |
| Troponin |
NA |
4467 (5.7) |
| Chest CT scan |
3 |
4523 (5.7) |
| BNP |
2 |
2739 (3.5) |
| Oxygen saturation |
1 |
No data |
ALevel 4 meant a referral to secondary/tertiary care.
BNP, brain natriuretic peptide; CT, computed tomography; NA, not applicable. |
| Table 3. Prevalence of conditions (concurrent diagnoses) among adults presenting with breathlessness (in order of prevalence)A |
| Disease |
N (%)B |
Related diagnostic |
| Lower respiratory tract infection |
54,322 (68.8) |
Chest X-ray, full blood count |
| Depression |
27,821 (35.3) |
NA |
| Anxiety |
23,410 (29.7) |
NA |
| Asthma |
24,062 (30.5) |
Spirometry |
| Coronary heart disease |
18,366 (23.3) |
ECG, echocardiogram, troponin |
| COPD |
17,914 (22.7) |
Spirometry, chest X-ray, thoracic CT scan |
| Heart failure |
14,517 (18.4) |
ECG, BNP, echocardiogram |
| Atrial fibrillation |
14,051 (17.8) |
ECG |
| Pulmonary embolism |
4005 (5.1) |
Thoracic CT scan |
| Hyperthyroidism |
2636 (3.3) |
Thyroid function test |
| Lung cancer (including suspect cases) |
1218 (1.6) |
Chest X-ray, thoracic CT scan |
| Rheumatic heart disease |
631 (0.8) |
ECG, echocardiogram, chest X-ray |
AAn individual can have more than one condition. These diagnoses were those noted by general practitioners in the dataset as of November 2021.
BPercentages calculated after excluding cases with incomplete details (n=2756).
BNP, brain natriuretic peptide; COPD, chronic obstructive pulmonary disease; CT, computed tomography; ECG, electrocardiogram; NA, not applicable. |
Discussion
Findings from the three studies support the acceptability and feasibility of our proposed diagnostic clinical algorithm in primary care, although modifications might be needed in its implementation to align with existing practice realities and enhance its uptake.
Previous studies have reported that over 70% of individuals with breathlessness had multimorbidity (two or more comorbid conditions), and 90% of those with COPD aged ≥45 years15 had at least one other chronic condition. Regardless, our analysis of the primary care dataset found that a relatively limited number of diseases were diagnosed in patients presenting with breathlessness, all of which could be further assessed using diagnostic tests in the proposed algorithm. The conditions identified were aligned with the major diseases identified in the National Breathlessness Survey of over 10,000 Australian adults, of which over 4000 had breathlessness, where they were asked to self-report the presence of a prior medical diagnosis of major conditions relating to breathlessness.1
The present study identified that, in general, GPs’ diagnostic tests referral align in practice with their theoretical ranking (ie pathology tests being most common). An Australian primary care study of 7255 breathlessness consultations between 2000 and 2009 reported that 26.3% ordered a radiological test and 62.4% a pathology test, which is a similar pattern to our findings.16 There was moderate agreement across the diagnostic tests between the GPs, but a wide IQR for the ranking of many diagnostic tests.
However, the present study highlights a significant gap between the tests GPs order and the diagnoses made. For example, although 30.5% and 22.7% of patients had an asthma and COPD diagnosis, respectively, across the six-year period, only 12% of diagnostic test referrals were for spirometry testing. This finding contrasts with previous studies in Australian general practice, which have reported over-testing as a significant problem but is in keeping with observations that 25–75% of tests are not supported by evidence or expert opinion.17 Overall, these results strongly support a need to develop pathways and services that would allow a more focused, efficient approach to assessing individuals presenting with breathlessness. They suggest that despite reflecting the prevalence of conditions associated with breathlessness in Australian practice, the proposed algorithm would have a greatly reduced efficacy if current diagnostic practices remain unchanged.
Spirometry is an essential tool in primary care for the assessment and management of breathlessness-associated conditions such as asthma and COPD. Our findings suggest that in patients presenting with breathlessness, spirometry was neither top of mind nor appropriately utilised, possibly leading to poorer patient outcomes. A Canadian population-based study showed that the use of spirometry testing to diagnose COPD patients in the ambulatory setting was associated with a 20% reduction in risk of death and admission to hospital.18 Although we note that the use of routinely collected data from primary care meant that there would be cases where diagnostic tests such as spirometry were conducted but not recorded as they were not digitised,19 previous studies in Australia and other settings have found similarly low rates of spirometry utilisation in primary care.20,21 As suggested in other studies,22,23 practical barriers such as knowledge gaps in technique and interpretation, along with poor access for referral services, must be addressed to solve this underutilisation of a vital diagnostic test.7,24 Solutions identified in prior studies include changes to current workflows; funding reform (as highlighted by GPs in our focus group to appropriately reimburse GPs for the cost of spirometry); and support for technical training, quality control and interpretation, including through decision support systems.25 Beyond this, the use of alternative tools that provide similar clinical utility to spirometry, such as forced oscillation technique (FOT) and breathomics, should be explored, especially in the current post-COVID-19 spirometry restart period.26,27
Similarly, there is low utilisation of BNP testing to screen for heart failure; a test recommended in clinical guidelines.28 Similar healthcare environments such as Europe and the UK provide public funding for this test to triage patients requiring cardiac imaging and cardiology assessment, as well as facilitate early diagnosis of heart failure in practice.29 Prior studies have shown that low N-terminal-prohormone brain natriuretic peptide (NT-proBNP) levels (≤125 pg/mL according to the European Society of Cardiology and ≤400 pg/mL according to the National Institute for Health and Care Excellence, UK) were both highly specific in excluding patients who did not have heart failure.30 However, a population-level study in the UK found that most patients (76.7% in 2017) with heart failure had no BNP test done prior to a heart failure diagnosis, suggesting that ongoing education and use of evidence in clinical pathways is vital.29 Our previous GP focus group found reimbursement and access to be major barriers to uptake in Australia.7 There is a need to support greater access to this high-yield test in primary care for which health economic studies have demonstrated cost savings when implemented widely.31,32
Independent of our study, another group in the UK also recently proposed a possible diagnostic clinical algorithm for chronic breathlessness,33 which included similar diagnostic tests and is being tested in 10 practices across Leicestershire. A major difference in their implementation is a focus on having all these tests and examinations completed within one month of a patient’s presentation for breathlessness rather than following a stepwise approach prioritising certain tests before others as with our proposed algorithm. If such an approach is implemented at scale, it could significantly raise the costs of assessment and increase pressure on the health system considering the prevalent nature of breathlessness in the community.
Beyond diagnostics, our previous study with GPs, non-GP specialists and allied health professionals identified a detailed medical history and physical examination as an essential part of a clinical algorithm to ascertain the cause of chronic breathlessness.7 This is especially so given that multimorbidity is present in over 70% of individuals with clinically relevant breathlessness. Further work could build upon this current study to develop a practical clinical algorithm that supports an efficient, evidence-based prioritisation of these diagnostic tests and leverages technologies such as machine learning. This would facilitate combinations of history, physical examination and diagnostic results from tests that are feasible in primary care to improve the accurate diagnosis in patients presenting with chronic breathlessness.
A limitation of the qualitative components of this study was that most GP participants were based in NSW and their responses might not be representative of the realities of practice in states and countries with different health system structures. However, we utilised results from a national primary care EHR dataset to complement the findings from the qualitative study, which includes practices from throughout Australia in its network. We do note, however, a limitation of the EHR study is that we cannot ascertain chronicity of the breathlessness presentation, and it was not possible to distinguish a new diagnosis from worsening of a pre-existing diagnosis. We reported the diagnoses for individuals at the end of the follow-up period, which includes the possibility that multiple visits, sometimes over years, can be required before a definitive diagnosis is achieved in an individual presenting with breathlessness.
Conclusion
The results of the three studies support the acceptability and feasibility of the proposed clinical algorithm in primary care, although modifications through testing it in practice will be needed to align with existing practice realities and enhance its uptake.