Case
A young girl, aged 20 months, presented with pubic hair growth and clitoromegaly. She was previously well with an uncomplicated perinatal history and normal neurodevelopment. There was no relevant past medical or family history.
Question 1
What is precocious puberty? What is virilisation?
Answer 1
Precocious puberty refers to the appearance of secondary sexual characteristics (eg breast growth, testicular enlargement, pubic hair) before the ages of eight years in girls and nine years in boys.1
Virilisation occurs when there is excess production of androgens leading to the development of exaggerated masculine features such as hirsutism (excessive male-pattern hair growth), pubic/axillary hair growth, clitoral or penile enlargement, adult-type body odour, acne, acceleration of growth, increased musculature, voice changes and irregular menstruation in postmenarcheal women.2
Case continued
This girl had features of virilisation with pubic hair (Tanner stage 2), mild facial acne and clitoromegaly. She did not have axillary hair, adult-type body odour, Cushingoid features or features of true (central) precocious puberty such as breast buds, acceleration in linear growth or vaginal discharge or bleeding. Gastrointestinal examination was within normal limits with no palpable abdominal masses. The rest of her clinical examination, including neurological (cranial nerve) examination, was unremarkable.
Question 2
What is the diagnostic approach to precocious puberty?
Question 3
What is the difference between central and peripheral precious puberty?
Answer 2
The diagnostic approach includes a clinical history, examination and investigations.
- Relevant history would include the following: details around the puberty changes, including breast development, vaginal bleeding, genital changes (testicular, penile, clitoral enlargement), pubic/axillary hair growth, adult-type body odour, acne and change in voice. For each of these changes, identify the age of onset and its progression. Growth acceleration or stalling of linear growth, weight gain. Exposure to exogenous sources of oestrogen or testosterone.
- A review of systems, including abdominal (abdominal mass, abdominal pain), neurological (visual changes, headaches) and symptoms of other hormone(s) deficiency/excess (eg symptoms of hypothyroidism). Family history, including onset of puberty, the heights of family members and endocrine, autoimmune and genetic conditions.
- Clinical examination would include the following (Table 1): a general examination, including growth parameters, syndromic features (eg Beckwith–Wiedemann, Li–Fraumeni), muscle/adipose tissue distribution and the skin (neurocutaneous stigmata, hyperpigmentation, acne, striae). A focused examination, including Tanner staging for puberty; signs of virilisation/excess androgen production (excess cortisol production: Cushingoid features; feminisation/excess oestrogen production [eg breast development]; other hormone(s) excess or deficiency); and abdominal and neurological examinations (eg abdominal mass, visual field deficit).3
| Table 1. Clinical presentations of hormonal excess6 |
| Hormone secreted in excess |
Clinical features |
% Childhood adrenocortical tumours |
| Cortisol |
Cushing syndrome |
3–8 |
| Aldosterone |
Conn syndrome |
<1 |
| Androgens |
Virilisation |
40–55 |
| Oestrogen |
Feminisation |
<1 |
| Mixed |
Mixed features |
45–50 |
In children who present with features of precocious puberty, differentiation between gonadotropin-dependent precocious puberty, gonadotropin-independent precocious puberty and benign pubertal variants without underlying pathology is crucial.1
First-line investigations include testosterone, oestradiol, dehydroepiandrosterone sulfate (DHEAS), 17-hydroxyprogesterone (17OHP), and basal and stimulated luteinising hormone (LH):follicle-stimulating hormone (FSH) ratio. A stimulated LH:FSH ratio is used to determine non-progressive precocious puberty versus central precocious puberty.4 A rise in testosterone and DHEAS can be due to normal pubertal development or suggestive of a secondary cause, whereas a rise in 17OHP can be suggestive of congenital adrenal hyperplasia (CAH) and requires further investigation by an adrenocorticotropin stimulation test.4
Imaging should include an ultrasound and computed tomography of the abdomen and pelvis to assess for possible tumours. In addition, a bone age assessment is performed to look for advances in height age correlating with precious puberty.4
Answer 3
Precocious puberty can be either central or peripheral in origin (Table 2). Central precocious puberty is gonadotropin-releasing hormone (GnRH) dependent and due to early activation of the hypothalamic–pituitary–gonadal axis. Peripheral precocious puberty is GnRH independent and due to the production of sex steroids from either endogenous or exogenous sources.
In women, activation of the hypothalamic–pituitary–gonadal axis leads to rising levels of oestrogen, which stimulate breast development (thelarche), the first clinical sign of true puberty. This is followed by a pubertal growth spurt and then onset of menarche around two years after the onset of thelarche. In men, the first clinical sign of true puberty is testicular enlargement. The pubertal growth spurt in men occurs later in the course of puberty than it does in women.6
| Table 2. Causes of central and peripheral precocious puberty5 |
| Central precocious puberty (GnRH-dependent) |
Peripheral precocious puberty (GnRH-independent |
- Idiopathic
- CNS trauma
- Tumours
- Infections (meningitis, encephalitis)
|
- Congenital adrenal hyperplasia
- Gonadal tumours
- Adrenocortical tumours
- McCune–Albright syndrome
- Primary hypothyroidism
- Exogenous exposure to sex steroids
|
| CNS, central nervous system; GnRH, gonadotropin-releasing hormone. |
Question 4
How do adrenocortical tumours present?
Answer 4
Most adrenocortical tumours present in childhood with signs/symptoms of hormonal excess. The clinical features of these tumours will differ depending on the hormone(s) being produced in excess.7
Case continued
Investigations showed elevated androgens (Table 3). Other hormone levels were within normal limits, with no signs of hypothalamic–pituitary–gonadal axis activation, hypercortisolism, hyperaldosteronism or hypothyroidism. Computed tomography imaging of the abdomen and pelvis showed a well-defined left adrenal mass measuring 57 mm × 45 mm × 46 mm with no features of invasion or distant metastatic spread (Figures 1 and 2). A left adrenalectomy was subsequently performed without any complications. Postoperatively, the elevated androgen levels rapidly normalised (Table 3) and there has been regression of the virilisation.
| Table 3. Hormone concentrations pre- and postoperatively in the clinic case |
| |
Preoperatively |
Postoperatively |
Reference intervals |
| 3 days |
2 months |
5 months |
| Adrenal: androgens |
| Testosterone (nmol/L) |
8.4 |
0.7 |
<0.5 |
<0.3 |
<0.5 |
| DHEAS (µmol/L) |
41 |
0.8 |
0.2 |
<0.5 |
<1.0 |
| Androstenedione (nmol/L) |
2.3 |
0.7 |
<0.4 |
0.2 |
0.1–0.7 |
| Adrenal: glucocorticoid |
| ACTH (ng/L) |
10 |
38 |
26 |
21 |
10–50 |
| Cortisol (nmol/L) |
130 (at 4 pm) |
303 (at 9.40 am) |
197 (at 8.30 am) |
284 (at 9.15 am) |
60–570 |
| Glucose (mmol/L) |
4.4 |
|
4.8 |
4.1 |
3.0–7.8 |
| Adrenal: mineralocorticoid |
| Aldosterone (pmol/L) |
92 |
|
|
|
100–1500 |
| Aldosterone/renin ratio |
<1 |
|
|
|
<55 |
| Sodium (mmol/L) |
136 |
|
139 |
138 |
133–144 |
| Potassium (mmol/L) |
4.2 |
|
4.8 |
4.6 |
3.9–5.6 |
| Hypothalamic–pituitary–gonadal axis |
| LH (U/L) |
1 |
|
|
|
<6 |
| FSH (U/L) |
2.4 |
|
|
|
<10 |
| Oestradiol (pmol/L) |
15 |
|
|
|
<100 |
| Thyroid axis |
| TSH (mU/L) |
2.6 |
|
|
|
0.7–5.9 |
| FT4 (pmol/L) |
14 |
|
|
|
8.7–16 |
| Tumour markers/genetics |
| β-hCG (IU/L) |
0.1 |
|
|
|
0.1–0.6 |
| TP53 gene |
|
|
|
No pathogenic variants detected |
|
| SNP array analysis |
|
|
|
Normal female molecular karyotype |
|
| ACTH, adrenocorticotropic hormone; β-hCG, beta human chorionic gonadotropin; DHEAS, dehydroepiandrosterone sulphate; FSH, follicle-stimulating hormone; FT4, free thyroxine; LH, luteinising hormone; SNP, single nucleotide polymorphism; TP53, tumour protein 53; TSH, thyroid-stimulating hormone. |
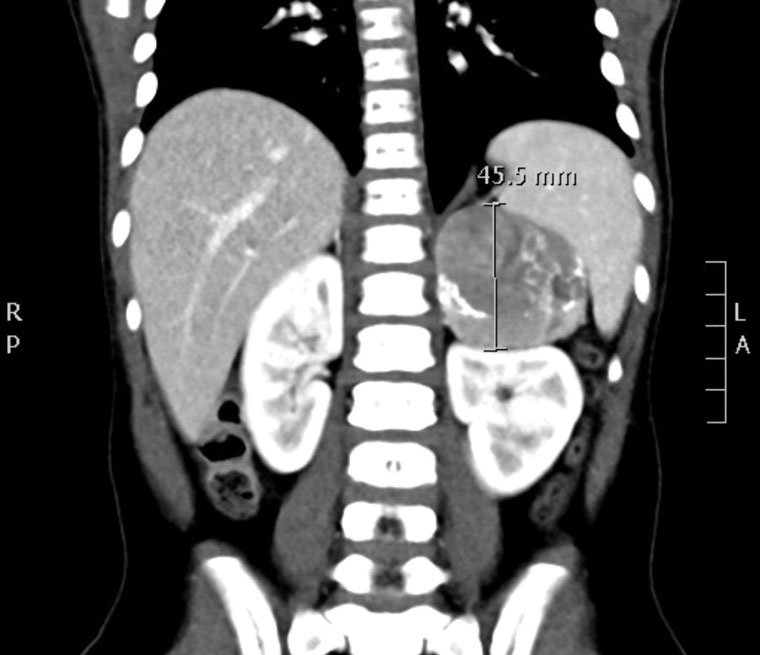 Figure 1. Computed tomography showing a well-defined left-sided lesion displacing the kidney inferiorly
Figure 1. Computed tomography showing a well-defined left-sided lesion displacing the kidney inferiorly
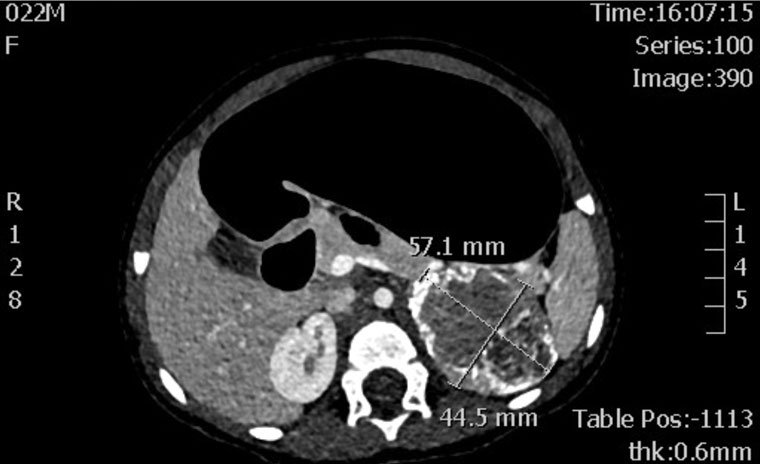 Figure 2. Computed tomography showing a well-defined left-sided lesion with soft tissue and calcific densities.
Figure 2. Computed tomography showing a well-defined left-sided lesion with soft tissue and calcific densities.
Key points
- It is important to distinguish central versus peripheral causes in precocious puberty with meticulous history, examination and investigations.
- Any signs of virilisation in a young child need to be investigated further.
- Adrenal tumours should be considered in cases of peripheral precocious puberty.