Otitis externa (OE) is a very common presenting complaint to both the general practitioner (GP) and the ear, nose and throat (ENT) specialist, with a lifetime risk of approximately 10%.1 OE is colloquially referred to as ‘swimmer’s ear’ as it is five-fold more prevalent in swimmers.1 Factors such as a temperate environment, humid climate, canal trauma, narrow external auditory canals (EACs), EAC obstruction, radio/chemotherapy and immunosuppression predispose an individual to OE.2
The year-round temperate and humid climate of Queensland, Australia, makes it perfectly suited for OE.3,4 In a survey conducted with 34 Queensland GPs, over 94% reported seeing OE at least 10 times per year, with 47.2% reporting seeing >30 cases.5 Treatment consists of mechanical lavage and topical antimicrobials.5 In Australia, the first-line treatment is dexamethasone/framycetin/gramicidin (DFG) combination ear drops, with only severe cases warranting oral antibiotics.6–8
The rationale for DFG drops is based on previous international literature that implicates Pseudomonas aeruginosa (PA) and Staphylococcus aureus (SA) as common pathogens.9 However, diversification might occur during seasonal variations; in summer, PA has a greater incidence due to higher average temperatures, increased water contact and greater rainfall.10 Demographics such as age and sex might also add to the variation of OE microbiota across the population.11
There is a large gap in data regarding pathogens responsible for OE in Australia. Most microbiological data originates from North America and Europe. We sought to explore the common pathogens causing OE in Queensland and to investigate our hypothesis that a variation in pathogens would be seen in different geographic locations with identifiably different climates. In addition, we wished to explore the hypothesis that there would be a variation in pathogens based on season, patient age and First Nations status.
Study aim
The primary aim of this study is to identify the most common pathogens implicated in OE that warrant ear swab culture in Queensland, Australia.
The secondary aims are to assess the effects of the following variables on the pathogen cultured:
- Season: summer (October to March) compared to winter (April to September)
- Location: coastal (within 50 km of a geographical coastline) compared to inland (greater than 50 km from a geographical coastline)
- Age: under age 18 years (child) compared to older than 18 years (adult)
- First Nations status: First Nations individuals compared to non-First Nations individuals.
Methods
Retrospectively, 1979 microbiology swabs sent to Pathology Queensland from hospitals statewide were retrieved from 1 January 2022 to 31 December 2022. The primary pathogen cultured, the specimen type, the date of collection and the patient demographics were retrieved.
A total of 232 specimens were excluded based on the specimen site information (eg abscess, pustule and lesion). Twenty-seven specimens were excluded due to the postcode being ‘null’ and 32 specimens because the postcodes were outside Queensland.
The following definitions were established to allow the classification of the variables:
- Summer and winter were classified according to a six-month split of the highest temperatures and lowest temperatures in Queensland. In summer (October to March), the average temperature ranged between 19.1 and 28.23°C, and the average humidity at 3 pm was 57%. In winter (April to September), the average temperature ranged between 12.3 and 22.3°C and the average 3 pm humidity was 49%.3,4
- Patients were classified according to age, with those aged ≥18 years considered adults and those aged <18 years children.
- Coastal and inland locations were determined based on the postcode listed on the individual specimen. With the use of online mapping, each postcode that was greater than 50 km from a coastline was classified as inland. Those that were within 50 km of a geographical coastline were classified as coastal.
Statistical analysis
Multivariate analysis was performed using Statistical Package for the Social Sciences (SPSS) Version 29 (IBM Corp., Armonk, NY, USA). To allow a meaningful comparison, only the eight most prevalent organisms were analysed, accounting for 1559 of 1688 (92.4%) specimens.
Ethics approval
Ethics approval was attained from the Royal Brisbane and Women’s Hospital Human Research Ethics Committee in January 2023 (approval EX/2023/MNHA/92667). This approval encompasses the entirety of the Pathology Queensland network.
Results
Demographics
Of the 1688 specimens analysed, 43% (726) were from female and 57% (959) from male patients, with gender not disclosed for <0.2% (3); 60% (1014) were classified as adults and 40% as children (674). First Nations individuals comprised 22.75% (384) and non-First Nations individuals 78.25% (1304) of the specimens analysed. Of the specimens for First Nations People, 45% (172) were classified as adults and 55% (212) as children. In terms of location, 86.3% (1457) were from coastal and 13.7% (231) from inland locations. Finally, 53% (888) of specimens were cultured in summer and 47% (800) in winter.
Pathogen distribution
Addressing the primary aim, the most prevalent pathogen cultured in the external ear in Queensland was PA at 37.9% (640), followed by SA at 22.5% (380), Candida spp. (CS) at 13% (219), Streptococcus pyogenes (SPy) at 6.7% (112), Haemophilus influenzae B (HIB) at 4.1% (69), Aspergillus spp. (AS) at 3.4% (58), Streptococcus pneumoniae (SPn) at 2.8% (48) and Fungus spp. (FS) at 2% (33) (Figure 1). All other pathogens combined comprised 7.6% (129) of the pathogen cultured in the external ear in Queensland (Appendix 1).
Comparing summer and winter, there was a decrease in the percentage of PA cultured from 43% to 33% (Figure 2). The incidence of SA, CS, SPy, AS and FS remained largely equivocal. HIB increased from 3% to 6% and SPn increased from 1% to 5%.
Looking at the location data, there was a reduction in PA cultured from 40% down to 32% between coastal and inland (Figure 3). There was an increase in SA from 21% to 32%, with an absolute difference of 11%. This was reflected as the most common pathogen in coastal locations being PA and in inland locations SA. CS decreased from 13% in coastal regions to 10% in inland regions. SPy, AS, HIB, FS and SPn remained largely equivocal.
Adults and children tended to grow different pathogens, with PA again being the most prevalent at 43% in adults and 31% in children (Figure 4). SA was seen in 20% of adults and 26% of children. CS was cultured in 20% of adults and 6.2% of children. SPy was cultured in 0.7% of adults’ specimens and 15.6% of children’s. AS was cultured in 5.5% of adults’ samples and 0.3% of children’s. HIB was seen in 0.5% of adults and 9.5% of children. FS was seen in 2.3% of adults and 1.5% of children. SPn was seen in 2.1% of adults and 3.7% of children.
When examining the difference between First Nations people and non-First Nations individuals, PA was 35% in First Nations people and 39% in non-First Nations individuals (Figure 5). SA, AS and SPn were not significantly different. CS was 11% in First Nations people and 14% in non-First Nations individuals. SPy was 8% in First Nations people and 6% in non-First Nations individuals. HIB was 8% in First Nations and 3% in non-First Nations individuals. FS was 0.5% in First Nations people and 2.4% in non-First Nations individuals.
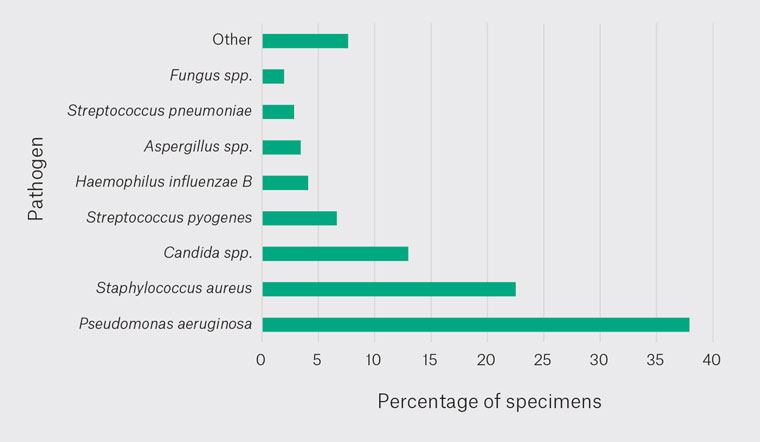
Figure 1. Pathogen prevalence.
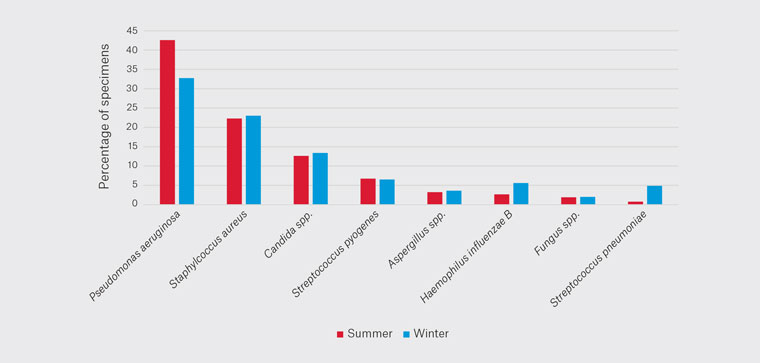
Figure 2. Seasonal variation of pathogens cultured.
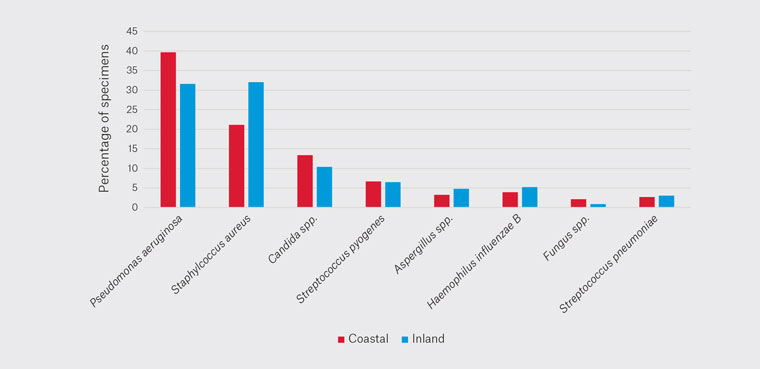
Figure 3. Locational variation of pathogens cultured.
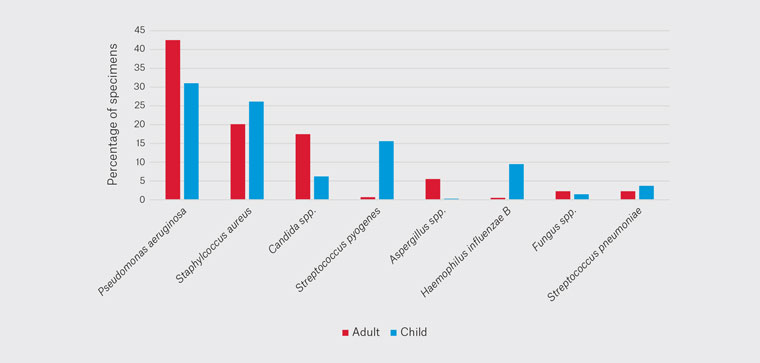
Figure 4. Variation of pathogens cultured with age.
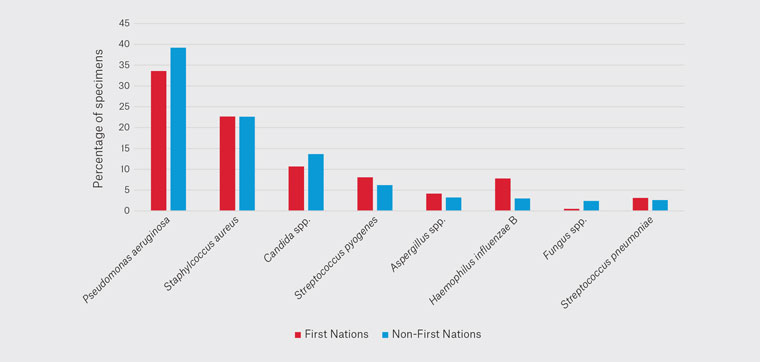
Figure 5. Variation of pathogens cultured based on First Nations status.
Discussion
In 2022, PA was the most prevalent pathogen causing OE in Queensland, being present in 37.9% of all ear swab cultures.
Our results are consistent with those published earlier for Far North Queensland by Nofz et al (2019), who identified that out of 5580 total specimens, 39% cultured PA, 22% SA, 18% CS, 10% SPy, 10% HIB, 8% AS and 7% SPn.12 The higher rates of respiratory pathogens (SA, SPy, HIB and SPn) are likely explained by the proportion of specimens from First Nations individuals, with Nofz et al having 60.3% specimens collected from First Nations people compared to only 22.7% in our dataset.
Interestingly, our results differ greatly from those published by Roland et al in 2002. In the North Americas, of 2048 individuals clinically diagnosed with OE, 98.3% cultured bacteria and only 1.7% cultured fungal species.13 In contrast, our data showed a bacterial prevalence of 80.1% and a fungal prevalence of 19.9%. Yet the percentage of PA cultured was near identical at 38%. Similarly, in the USA, OE clinical guidelines quote a prevalence rate of 20–60% for PA and 10–70% for SA, with fungal species being noted to be distinctly uncommon in acute OE.14 Furthermore, Weigand et al (2019) had similar results, finding that >90% of OE are caused by bacteria, with the prevalence of PA being 22–62% and SA 11–34%. They came to a similar conclusion that fungal acute OE was rare, noting that long-term antibiotic use, immunosuppression and diabetes mellitus increased an individual’s risk of OE.1
The higher rate of fungal pathogens cultured from specimens in our dataset is likely explained by the relatively higher humidity and average temperature compared to the Northern Hemisphere where much of the other available data are from. Higher humidity and temperature have been shown to lead to an increased incidence of fungal infections.15
Further analysis
To allow further analysis, 41 of 49 low-incidence (7.6%) pathogens were removed. A multivariate analysis revealed that the following were statistically significant: season (P<0.001), age (P<0.001), location (P<0.001) and First Nations status (P<0.001). Sex was not (P=0.362).
Three trends emerged, which remained consistent throughout the analysis. First, when compared to the overall results, there was a higher prevalence of SA (overall 23%) in both adults (33%) and children (35%) in inland regions, both of which were statistically significant (P<0.05). Similarly, for adults that lived within 50 km of a coastline, the rates of PA were higher: 48% compared to the overall 38%. A potential explanation for this might be related to access to swimming pools, rivers and beaches, as PA is a common pathogen in water.16
Most interestingly, there was a large discrepancy in the rates of fungal OE between adults and children. When the three analysed fungal species incidence was combined, this translated to 256 of 926 specimens (27.6%) for adults and 54 of 633 specimens (8.5%) for children: an over three-fold higher prevalence of fungal pathogens in adults than in children. The literature does not reveal a clear answer for this phenomenon. It might be attributable to fungal OE tending to occur in immunosuppressed, diabetic patients and those with a history of recurrent OE.1
Interpreting the results comparing First Nations people to non-First Nations individuals is challenging. The results will invariably be skewed from specimens collected for acute otitis media (AOM) and chronic suppurative otitis media (CSOM), rates of which are considerably higher in First Nations people.17
Confounding factors and limitations
The true incidence of OE is likely far higher than reflected here. Most GPs, emergency departments and ENT clinicians will not take a sample for microscopy, culture and sensitivity (MCS) unless there is reason to believe a patient will be non-responsive to first-line antimicrobial treatment. Conversely, the prevalence of pathogens in this dataset is demonstrated only in the same population of patients who are likely to present to medical services for treatment. Therefore, although this study might not fully represent patients with mild/subclinical OE, it is relevant to inform antibiotic guidance from the population illustrated here. Further, as these data are an aggregate of all specimens collected from the ear canal, as alluded to previously, there would invariably be specimens that were collected for AOM and CSOM. This makes the interpretation of respiratory pathogens, particularly in winter, more difficult. Similarly, the SA and SPy data need to be interpretated with caution, as both these pathogens are implicated in OE and otitis media. A further prospective exploratory study would be beneficial to ratify the trends identified in our study.
Clinical implications
At a population level, PA is common in Queensland, particularly in coastal regions during summer. Specific groups, especially in winter, might warrant MCS when PA is not the most common pathogen. Fungal OE in children is rare (<9%); as such, there should be a high level of clinical suspicion to commence antifungal ear drops. Conversely, SA is common, particularly in children living within inland communities, and this should be considered when selecting the appropriate antimicrobial ear drops.