Healthy skin is essential for overall health. Understanding the skin conditions that impact different populations provides a framework to develop appropriate resources and services.1 For Aboriginal families, this includes prioritising a holistic approach, integrating traditional health practices and acknowledging the connection of health to ancestry, country, culture, community and family.
To improve health outcomes for Australian Aboriginal people, and overcome structural racism embedded within mainstream healthcare, Aboriginal Community Controlled Health Organisations (ACCHO) were established during Australia’s Black Power movement.2 Through >140 member ACCHOs nation wide, this sector now delivers almost 3.1 million episodes of care per year and in 2020, collectively serviced >410,000 people.3 The lifetime health impact of ACCHO-delivered interventions is 50% greater than that of mainstream healthcare services, emphasising the strength of this sector in achieving meaningful outcomes.3
Although a heavy burden of skin infections affecting remote-living Aboriginal children has been documented, little is known about skin health of urban-living Aboriginal children.4 In Western Australia (WA), this is despite nearly 60% of all Aboriginal children and young people (CYP, aged <18 years) residing in urban settings (major cities and inner regional areas).5 Hospitalisation rates for skin infections are 10-fold higher in urban-living Aboriginal CYP compared with non-Aboriginal CYP; this is associated with an increased risk of serious complications including invasive infection, acute post-streptococcal glomerulonephritis (APSGN), acute rheumatic fever (ARF) and rheumatic heart disease (RHD).4,6 Yet, hospitalisation is likely an underestimate of skin infection burden, as most are managed in primary care.
Globally, atopic dermatitis (AD) or eczema, is the most common chronic inflammatory skin condition in CYP, with rising prevalence.7 AD is a risk factor for skin infections and adversely impacts general health, school performance and quality of life.4,8 AD and skin infections are more prevalent and severe among urban-living Indigenous CYP in high-income countries globally, as compared with non-Indigenous CYP.9 AD is the leading cause of skin disease burden (59%) in Aboriginal children aged <5 years and the second most common (33%) for children aged 5–24 years.10
Across all ages, skin disorders represent 16% of primary care consultations for urban-living Aboriginal patients, and 22% for remote-living Aboriginal patients.11,12 We aimed to investigate the burden and clinical characteristics of skin disorders affecting urban-living Aboriginal CYP in primary care.
Methods
Study design and setting
A retrospective cohort study was conducted at Derbarl Yerrigan Health Service (Derbarl), an urban ACCHO located on Whadjuk Noongar (Perth, WA) boodjar (land/place). The Noongar Nation are the traditional custodians of south-west WA and includes 14 clans/language dialects: Whadjuk refers to the Perth clan. The Noongar calendar includes six seasons indicated by flowering of local plants and insect/animal behaviours, following a Mediterranean climate (Figure 1).
Derbarl was established in 1973 and is the largest ACCHO in WA. It provides integrated primary health services across four sites to >15,000 Aboriginal patients annually, 30% of whom are CYP (Derbarl Yerrigan Health Service Business Information Unit, unpubl data). Derbarl utilises an Aboriginal health practitioner (AHP)-led model of care with patients receiving an age-appropriate assessment prior to a general practitioner (GP) consultation, excluding patients requiring urgent care or those presenting with an acute respiratory illness during the COVID-19 pandemic. For children aged <15 years, the AHP assessment includes skin and head lice examinations.
Eligible study participants were identified through an electronic medical record (EMR) search as those aged <18 years who attended a GP appointment at the central (East Perth) clinic over a 12-month period (01 October 2020 – 30 September 2021). Patients were excluded if they did not have a documented GP clinical interaction or if they attended for influenza/COVID-19 vaccination only (Figure 2). The 12-month audit period coincided with the COVID-19 pandemic, including three short COVID-19 lockdowns (February, April and July 2021). The Derbarl COVID-19 response included holistic COVID-19 care services for patients in isolation, with increased GP telehealth consultations.13 A monthly paediatric dermatology clinic commenced at the central Derbarl site in May 2021.
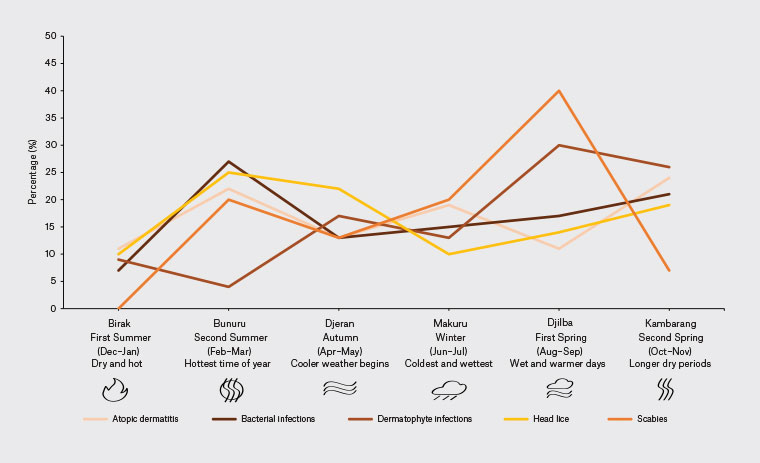
Figure 1. Noongar seasonal variation of dermatological disorders addressed in primary care.
Adapted from Barrow J, Nannup N (Uncle). Nyoongar seasonal calendar. Commonwealth of Australia, Bureau of Meteorology, 2016, www.bom.gov.au/iwk/calendars/nyoongar.shtml, with research and content by Barrow J and Uncle Noel Nannup, and with permission from Uncle Noel Nannup.
Data extraction, study measures and statistical analysis
All clinic presentations for eligible participants were retrospectively reviewed and data were collected by the lead author and dermatologist (BMR). Baseline demographic data were collected at first presentation: birth date, sex, Indigenous status, residential address and past history of AD, APSGN, ARF and RHD. The 2019 Modified Monash (MM) category was used to classify geographical remoteness, with urban-living defined as MM1 (metropolitan areas) or MM2 (regional centres).14
GP consultations were recorded as separate episodes of care and data extracted regarding service delivery (date, mode of consultation, AHP skin and head lice assessments) and clinical presentation, including details of all dermatological disorder(s) addressed (primary or secondary diagnoses). Dermatological disorders were classified into broad diagnostic categories (Appendix 1). For skin infections (bacterial, dermatophyte and viral), scabies and AD, data on clinical features, investigations and management were extracted. New bacterial skin infection (BSI) was defined as a clinical presentation for BSI occurring >30 days from index diagnosis (to exclude the possibility of counting scheduled reviews, relapses or treatment failure). Recurrent BSI was defined as more than one new BSI episode per 12 months.
Primary outcome measures related to skin disease burden among urban-living Aboriginal CYP were: (1) proportion (proportion of GP consults addressing a dermatological disorder, AHP skin assessments documenting ‘abnormal skin’, AHP head lice assessments documenting head lice); (2) incidence (cumulative incidence and incidence rate for new BSI); and (3) prevalence (contact prevalence for head lice, dermatophyte infections, scabies, viral skin infections and AD; lifetime prevalence for AD, APSGN, ARF and RHD). Secondary outcome measures included clinical features, seasonal variation, investigations and treatment of common skin disorders.
All data were entered into a REDCap database and analysed using R version 4.1.2 (R Foundation for Statistical Computing, Vienna, Austria). Descriptive statistics summarised patient demographics, episodes of care, dermatological disorders and clinical features. Proportion, incidence and prevalence calculations were performed (Table 1). GP consult data were used for analysis of all disorders except head lice, where AHP assessment data were used. The frequencies of categorical variables (age, sex, MM category) were compared between outcome categories (infections, infestations, AD) using Fisher’s exact test, and reported with odds ratios and 95% confidence intervals.
Ethics approval
Ethics approval was granted by the Western Australian Aboriginal Health Ethics Committee (HREC Ref No. 1059) and the University of Western Australia (File Reference – 2021/ET000536).
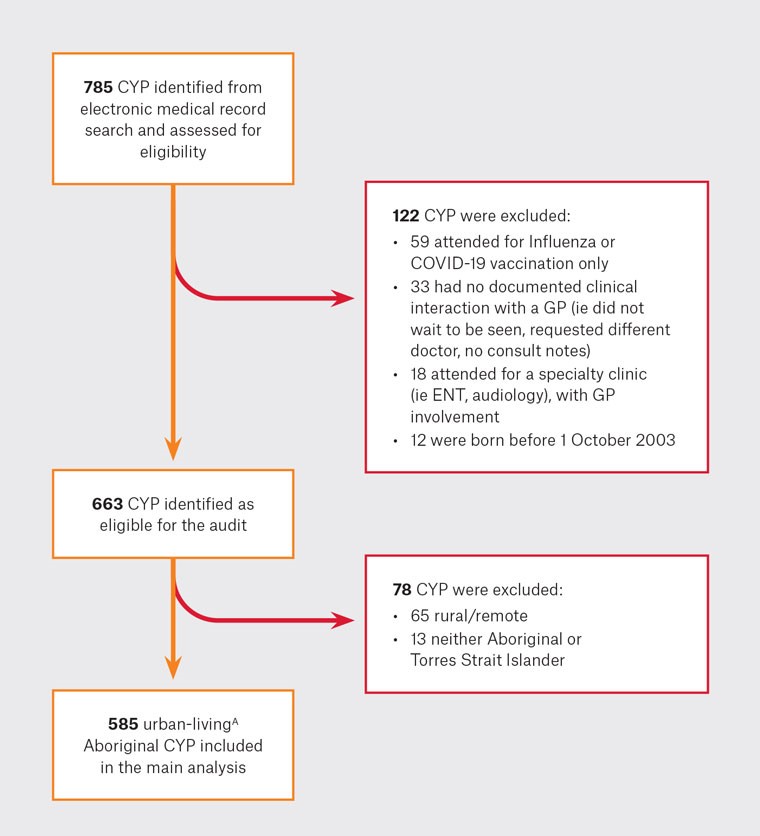
Figure 2. Participant selection.
AUrban-living defined as Modified Monash 1 (metropolitan areas) or Modified Monash 2 (regional centres).
CYP, children and young people (aged 0–18 years); ENT, ear, nose and throat; GP, general practitioner.
| Table 1. Definitions of numerators and denominators used in analysis |
| |
Numerator |
Denominator |
| Proportion |
|
|
| Proportion of GP consultations or AHP assessments |
Number of episodes of care for specified disorder |
Total number of episodes of care |
| Incidence |
| Cumulative incidence |
Number of patients with a new episode of BSI over 12 months |
Population at risk (total number of patients) |
| Incidence rate |
Sum of all new episodes of BSI over 12 months |
Child-years at riskA |
| Prevalence |
| Contact prevalence |
Number of patients with ≥1 contact for a specified skin disorder over 12 months |
Population at risk (total number of patients) |
| Lifetime prevalence |
Number of patients with an ever-recorded specified disorder in the EMR |
Population at risk (total number of patients) |
AThe at-risk period is the period that a patient was not recorded having a BSI. We utilised a 30-day washout period for a new BSI and patients were able to contribute multiple events during the study period (recurrent BSI).
AHP, Aboriginal health practitioner; BSI, bacterial skin infection; EMR, electronic medical record; GP, general practitioner. |
Results
Study population
A total of 785 patient EMRs were screened and 585 urban-living Aboriginal CYP were included in the analysis (Figure 2). The median age was seven years (IQR 3–12), 50% were female and 99% resided in MM1. There were 989 episodes of care recorded for 585 CYP, equating to 1.69 presentations (range: 1–10) per CYP over 12 months (Table 2).
Burden of skin disorders
Proportion results
At least one dermatological disorder was addressed in 26% (259/989) of GP consultations, and at least one skin infection in 14% (139/989). Face-to-face consultations accounted for 95% (939/989) of episodes of care and were associated with a three-fold (OR 2.7, 95% CI 1.13–7.86) likelihood of addressing a dermatological disorder (253/939, 27%) compared with telephone consultations (6/50, 12%). In total, 284 dermatological disorders were documented in 989 episodes of care, with BSI, dermatitis and dermatophyte infections accounting for 30% (84/284), 18% (50/284) and 8% (23/284), respectively (Figure 3).
AHP skin assessments occurred before 72% (673/939) of face-to-face GP consultations, with ‘abnormal skin’ documented in 21% (196/939). ‘Rashes’, ‘infected sores’, ‘dry areas’, ‘eczema’ and ‘healing sores’ accounted for 27% (53/196), 25% (49/196), 15% (30/196), 14% (27/196) and 12% (24/196), respectively. AHP head lice assessments occurred before 65% (606/939) of face-to-face GP consultations, with head lice detected and treated in 9% (88/939).
Incidence results
There were 82 incident cases of BSI affecting 74/585 urban-living Aboriginal CYP over 12 months (13% cumulative incidence; 95% CI 10–16%), which was highest in children aged 1–12 years (OR 2.44; 95% CI 1.28–4.98) affecting 15% in this age group (61/397). The incidence rate of all BSI in the 12-month follow-up was 142/1000 child-years-at-risk. Recurrent BSI affected <1% (5/585; 95% CI 0.3–2%).
Prevalence results
The 12-month contact prevalence of head lice, AD, dermatophyte infections, viral skin infections and scabies were 18% (74/419; 95% CI 14–22%), 6% (33/585; 95% CI 4–8%), 4% (22/585; 95% CI 2–6%), 3% (15/585; 95% CI 1–4%) and 2% (13/585; 95% CI 0.06–1%), respectively. Head lice was most prevalent in children aged 5–12 years (OR 2.49; 95% CI 1.44–4.35) affecting 25% in this age group (46/183), AD in children aged 0–4 years (OR 2.28; 95% CI 1.06–4.98) affecting 9% (18/208), and dermatophyte infection in children aged 1–4 years (OR 3.21; 95% CI 1.24–8.48) affecting 7% (12/165).
The lifetime prevalence of AD was 16% (94/585; 95% CI 13–19%), whereas ARF, RHD and APSGN were 0.9% (5/585; 95% CI 0.03–2%), 0.2% (1/585; 95% CI 0.008–1%) and 0.2% (1/585; 95% CI 0.008–1%), respectively.
| Table 2. Episodes of care by age group |
| |
TotalA |
Age group (years)A |
| 0 to <1 |
1 to <5 |
5 to <13 |
13 to <19 |
| n (%)B |
585 |
43 (7) |
165 (28) |
232 (40) |
145 (25) |
| Episodes of care per participant |
1–3
4–6
7–10 |
540 (92)
36 (6)
9 (2) |
42 (98)
0 (0)
1 (2) |
151 (92)
12 (7)
2 (1) |
211 (91)
16 (7)
5 (2) |
136 (94)
8 (5)
1 (1) |
| Total episodes of careB |
989 |
74 (7) |
292 (30) |
385 (39) |
238 (24) |
An (%), percentages are column percentages.
BRow-wise percentages provided for this row. |
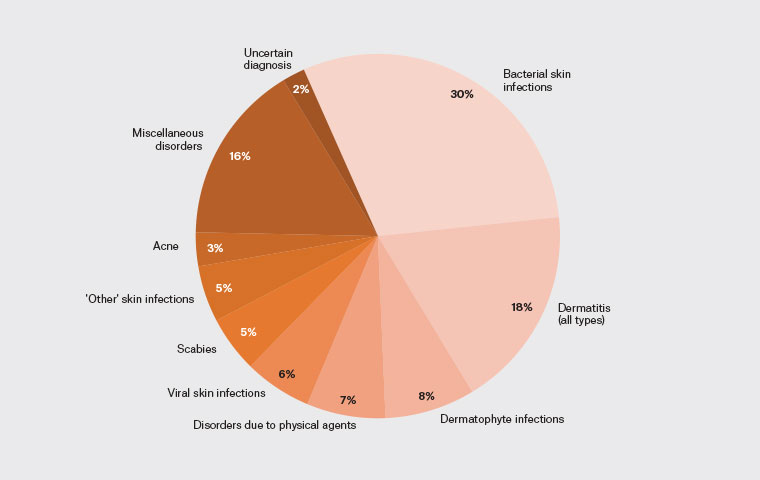
Figure 3. Proportion of dermatological disorders addressed in primary care.
Clinical characteristics of skin disorders
Seasonal variation
Of face-to-face consultations, BSI, AD, dermatophyte infections, viral skin infections and scabies were addressed in 9% (84/939), 4% (37/939), 2% (23/939), 2% (16/939) and 2% (15/939), respectively. The Noongar seasonal variation of skin disorders is shown in Figure 1; with BSI (25%) and head lice (27%) peaking in Bunuru (February/March), AD (24%) in Kambarang (October/November) and dermatophyte infections (30%) and scabies (40%) in Djilba (August/September).
Clinical features
Impetigo was the most frequent BSI subtype occurring in 63% (52/82) of affected children, followed by carbuncles/furuncles in 15% (12/82). Lower limbs (32%), upper limbs (23%) and face (20%) were most commonly affected. No precipitating event was documented in 60% (49/82), whereas skin injury/trauma (17%), AD (5%), scabies (5%) and head lice (4%) most frequently precipitated BSI.
Of ‘all dermatitis’ cases, AD accounted for 74% (37/50) and irritant contact dermatitis 26% (13/50). Exposed skin was most commonly affected (43%) in dermatophyte infections, followed by covered skin (35%) and the scalp (13%). Molluscum contagiosum accounted for most viral skin infection presentations (38%), followed by hand-foot-and-mouth disease (25%) and non-specific viral exanthems (19%).
Investigations
Wound swabs were collected in 11% (9/82) of incident BSI cases, with Staphylococcus aureus cultured in 100% (9/9) and Streptococcus pyogenes in 22% (2/9). Nail clippings were collected from one of two patients with onychomycosis. Hair plucks were not collected in the three patients with tinea capitis.
Treatment
Systemic antibiotics were commenced in 83% (68/82) of incident BSI cases; most frequently Flucloxacillin (34%, 23/68), Cephalexin (32%, 22/68) and Trimethoprim/Sulfamethoxazole (18%, 12/68). Topical mupirocin ointment and antiseptic washes were both prescribed in 11% (9/82). One patient (1.2%, 1/82; 95% CI 0.06–7%) was referred to the tertiary children’s hospital for inpatient management of severe BSI.
Regarding AD consultations, 59% (22/37) were prescribed topical corticosteroids (TCS), 41% (15/37) emollient, 24% (9/37) soap-free wash and 5% (2/37) wet wraps. Referral to the ACCHO-embedded paediatric dermatology clinic was made for 27% (9/33; 95% CI 14–46%) of CYP with AD.
For dermatophyte infection, topical azole therapy (cream, shampoo, powder) was prescribed in 61% (14/23), combination topical steroid-antifungal in 22% (5/23) and terbinafine cream in 9% (2/23). No systemic antifungal therapy was prescribed for nail or scalp dermatophyte infection, but one patient with tinea capitis was referred to the ACCHO-embedded paediatric dermatology clinic.
All 15 patients with scabies were treated with Permethrin 5% cream.
Discussion
In the first study to investigate the burden and clinical characteristics of skin disease in urban-living Aboriginal CYP in a primary care ACCHO clinic, we found:
- dermatological disorders were addressed in 27% of face-to-face GP consultations, with a high burden of skin infections and dermatitis
- AHP skin screening aided GP assessment by identifying ‘abnormal skin’ in 21% of face-to-face GP consults, and facilitated head lice treatment in 9%
- BSI affected one-in-eight CYP, yet recurrent BSI affected <1% and only 1% of BSI episodes required hospitalisation
- ACCHO-embedded specialist dermatology care was sought for 27% of CYP with AD.
At least one dermatological disorder was addressed in 27% of face-to-face GP consultations in our study; these were most frequently infections and dermatitis. Data for comparison for non-Aboriginal urban-living CYP is limited; however, two studies provide data for all urban-living Australian CYP. In the first, 20% of consultations for CYP (aged 0–19 years) in two general practices concerned a dermatological problem; most commonly eczema/dermatitis (31%) and infections (15%).15 In the second study that analysed adolescents (aged 10–19 years), skin problems were managed in 24% of consultations; with infections, acne and dermatitis most frequent.16 Although the proportion of dermatological disorders addressed in our study is similar to that described for all urban-living Australian CYP, the burden of infections is higher, with 14% of all GP consultations addressing at least one skin infection, compared with 3–6% for all urban-living Australian CYP.15,16 Our results are similar to those reported for remote-living Aboriginal children (aged <7 years), where skin infections accounted for 16–18% of primary care presentations.17,18
Our finding that more than one-quarter of GP assessments addressed a dermatological disorder is supported by the AHP-led model of care provided, where AHP skin assessments identified ‘abnormal skin’ in 21% for GP assessment. AHP head lice assessments occurred prior to 65% of face-to-face GP consultations, providing opportunistic treatment in 9% to potentially reduce BSI; our study also indicated head lice-precipitated BSI in 4%. Our study suggests an 18% prevalence of head lice, consistent with previous reports about urban-living Aboriginal CYP.19 It is important to note that the background prevalence of head lice in non-Aboriginal urban-living CYP is unknown as the AHP-delivered assessment is unique to the ACCHO setting, hence head lice might go undiagnosed in mainstream primary care.
We found one new BSI for every eight CYP attending the urban ACCHO over 12 months, peaking in Bunuru (February/March), which is consistent with descriptions of increased BSI in late summer in hot, dry environments.6 Our BSI incidence rate of 142/1000 child-years-at-risk in primary care is much higher than linked hospitalisation data (1996–2012) for urban-living Aboriginal CYP in WA (20.8/1000 child-years-at-risk).6 Primary care ACCHO consultations provide an opportunity to prevent hospitalisation through early detection and treatment of skin infections by the AHP and GP. Only 1/82 (1.2%) BSI episodes required hospitalisation, which compares favourably with a New Zealand study of Indigenous children that estimated there to be 14 primary care cases of BSI for every one case requiring hospitalisation.20 Recurrent BSI affected <1% (5/585) of CYP, which is less than that reported in a 2000–01 carer-completed survey of urban-living Aboriginal children where 7.1% (720/10,200) described ‘recurring skin infections such as school sores or scabies’.21
We report a 16% lifetime prevalence of AD, similar to the 19% lifetime prevalence of parent-reported ‘eczema ever’ described in a community cohort of urban-living Aboriginal CYP.19 These results add to the growing knowledge of AD burden among urban-living Aboriginal CYP, where ‘eczema ever’ has been reported in 13–25% (for children aged <6 years).22,23 In our study, the 12-month contact prevalence of AD was significantly less (6%) than the lifetime prevalence (16%). This reflects the chronic nature of AD and that once diagnosed and treatment has been initiated, patients might not need to visit their GP as often due to good control, improvement in disease severity (as can occur later in childhood) or if they are receiving specialist care. Alternatively, reduced GP visits might not correlate with better control, but perhaps reflect a lack of understanding of the chronic nature of AD, which benefits from regular physician review to ensure optimal control. In our study, AD presentations peaked in Kambarang (October/November), which is consistent with data from Korea, the Netherlands and US that show increased AD symptoms and/or healthcare utilisation in Spring, reflecting increasing ambient temperatures and/or the pollen season.24–26 In the Netherlands, children with flaring AD in the pollen season more often had hay fever at a younger age and a dark skin type.25
The 12-month contact prevalence for dermatophyte infections was 4%, significantly less than the 19% point-prevalence of dermatologist-diagnosed dermatophyte infections (including 10% point-prevalence of tinea capitis) in urban-living Aboriginal CYP at a WA community skin screening event; this suggests possible underdiagnosis in primary care.19 We advise a high index of suspicion for tinea capitis in CYP noted to have both scalp scaling and hair thinning (82.1% sensitivity) or scalp scaling alone (60% sensitivity).27 Likewise, tinea affecting any skin or nail site should prompt thorough scalp examination.28 In our study, dermatophyte infections peaked in Djilba (August/September), alongside the scabies peak. Although scabies has been reported to predominate in winter due to enhanced mite survival and closer living encouraged in cooler temperatures, the same predominance of dermatophyte infection has not been described; however, the element of closer living might be relevant.29
Evidence-based guidelines, such as the Antibiotic and Dermatology Therapeutic Guidelines (www.tg.org.au) and the National Healthy Skin Guidelines, are helpful resources for Australian primary care providers; the latter focussing on Aboriginal children and communities.30 Our assessment of GP documented treatments found that, overall, these guidelines were well adhered to. We did note frequent (22%) prescription of topical steroid–antifungal combinations for tinea corporis, a practice we discourage due to the potential of the steroid component to worsen tinea, cause skin atrophy and striae, and contribute to emerging public health concern of antimicrobial-resistant tinea infections.31 We also noted an absence of fungal culture and systemic antifungal prescription for tinea capitis, where species identification and prescription of oral terbinafine is recommended first-line treatment; however, referral was made to the ACCHO-embedded paediatric dermatology clinic.32 Referral to this clinic was also made for over one-quarter of CYP with AD, supporting the value of this service in providing prompt and accessible care.
Limitations include retrospective design and potential for information bias as data were obtained from patient EMRs. The data collection period coincided with the COVID-19 pandemic. During this time, health-seeking behaviours were impacted, telehealth consultations increased and the clinician’s approach to face-to-face consultations was adjusted to minimise patient contact time, resulting in reduced opportunistic skin examinations and potential underdiagnosis of skin disease. In addition, it is a single site study, which limits generalisability and external validity, and might have had a reduced number of infant assessments as young families often access the peripheral Derbarl clinics. Beyond Derbarl, urban-living Aboriginal CYP with a health complaint in metropolitan Perth might attend a non-ACCHO GP practice or one-of-four emergency departments that assess children. Univariate analysis was used to compare frequencies of categorical variables between outcome variables, with the inherent risk of confounding in these relationships. The findings of this study must be considered in the context of these sources of potential bias.
This is the first study to investigate skin health in urban-living Aboriginal CYP presenting to primary care. The results indicate that dermatological disorders account for a significant proportion of the ACCHO GP workload, with a high burden of skin infections and dermatitis identified. BSI affected one-in-eight urban-living Aboriginal CYP presenting to this ACCHO, yet recurrent BSI affected <1% and only 1% of BSI episodes required hospitalisation; this suggests early and appropriate treatment minimised recurrence and hospitalisation. We present a culturally secure, multidisciplinary skin health assessment model involving AHPs, GPs and dermatologists within an urban ACCHO, which can be emulated throughout the nation to help achieve optimal outcomes for patients and their families.