The current epidemic of chronic conditions and multimorbidity threatens to overwhelm the capacity of health systems in Australia and across the developed world. The biomedical model that has delivered strongly in the treatment of acute conditions has largely failed in the context of chronic care. Addressing chronic conditions as multiple ‘single diseases’, rather than with an integrated approach, brings health system inefficiency and suboptimal outcomes for individuals.
This article is written by clinicians from a tertiary multidisciplinary chronic pain team. Our perspective reflects the rarity of seeing a patient with pain but no other chronic conditions at the service. In addition to chronic pain, a typical patient might present with depression, type 2 diabetes, obesity, asthma, hypertension and gastroesophageal reflux disease. The perspective is also informed by the view that the whole-person approach used for the treatment of chronic pain at the service has relevance to multiple chronic conditions.1
A cumulative strain model (Figure 1) is proposed that recognises shared biological mechanisms underlying many chronic conditions. This underlines the untapped potential for generalisable treatments, particularly with respect to behavioural and lifestyle interventions. The model also acknowledges a select role for evidence-based, condition-specific biomedical treatments.
The aim of this article is to highlight common mechanisms underpinning chronic conditions and prompt consideration of how these mechanisms might be targeted therapeutically in primary care. Recognition of commonalities means that chronic pain need not be treated in isolation, and active behavioural approaches might have benefit for multimorbid conditions.
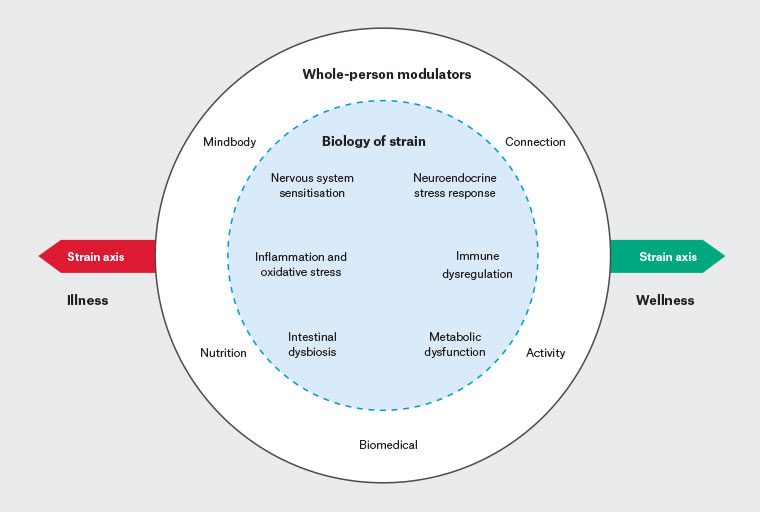
Figure 1. A cumulative strain model for chronic pain and multimorbidity. Biological mechanisms of strain are shown in the inner circle with whole-person modulators in the outer circle. The strain axis illustrates potential shift towards illness or wellness.
Epidemic of chronic conditions and multimorbidity
In Australia in 2020–21, 56.5% of adult women and 49.5% of men had one or more chronic conditions; the most common was mental and behavioural (20.1%), followed by back problems (15.7%) and arthritis (12.5%).2 Many people with chronic conditions had multimorbidity, defined as more than one condition at the same time. For example, 70.7% of those with arthritis and 64.4% of those with back problems had multimorbidity.2
Epidemiology of chronic pain
Chronic pain is defined as persisting or recurring pain lasting longer than three months.3 In 2018, it was estimated that one in five Australians experienced chronic pain, with a cost of $139 billion, including productivity losses, as well as reduced quality of life.4 Women, older people, rural residents and those with low socioeconomic status are more likely to experience chronic pain.5
Explanatory pain models
Explanatory models for chronic pain have been extensively reviewed.6 Such models have value in unifying clinician language, communicating with consumers and focusing on research. In explaining chronic pain,7 a biopsychosocial approach is widely accepted. From a biological perspective, there is a field of research endorsing the importance of nervous system sensitisation and loss of descending inhibition.8 There is recognition of the immune system and the role of glial cell activation in the neural sensitisation process.9 These mechanisms are known to play a critical role in the persistence and intensity of pain experienced in various chronic pain disorders, including those with clear peripheral drivers, such as rheumatoid arthritis and osteoarthritis. Notably, however, in existing pain models, there is a lack of recognition of the strong association with multimorbidity and the commonality of underlying mechanisms and treatments; hence the need for a broader conceptualisation.
Cumulative strain model
A cumulative strain model relevant to chronic pain and multimorbidity is illustrated in Figure 1. High-level biological mechanisms of strain are shown in the inner circle, with ‘whole-person’ modulators10 in the outer circle. Mindbody, connection, activity, nutrition and biomedical modulators can worsen or improve biological expression of strain. The model allows for the whole system to shift along the strain axis over time towards either wellness or illness.
The intention of this model is to provide clinicians with a broader patient-centred approach that has utility in framing diverse therapeutic interventions for pain and multimorbidity. The model might have particular salience in primary care.
Differentiating stress and strain
Differentiation between stress as the source of threat and strain as the impact of that threat upon the individual11 is useful in the context of chronic conditions. It recognises that people respond to the same stress with different degrees of strain. Therapeutically, although stress might be difficult to change, strain (or present-moment response) is more modifiable.
The background level of strain varies across the life course. The experience of stressors during early childhood can embed changes in capacity to respond. This occurs via the nervous, immune and endocrine systems as ‘allostatic load’,12 a term that is analogous to cumulative strain. In framing an effective approach to multimorbidity, it is critical that clinicians recognise the contribution of adverse childhood experiences to cumulative strain. This cumulative strain, in turn, predisposes to the development of chronic conditions later in life.12,13
Biology of strain
In some situations, the development of a chronic condition follows acute threat or injury. There is initial immune system activation with acute inflammation and oxidative stress,9 a neuroendocrine stress response and linked metabolic change. Typically, these homeostatic processes resolve as the threat passes and the body heals. However, if the threat or perception of threat continues, homeostasis might not be restored and a chronic condition develops. The transition from acute to chronic pain is an example.
There are other situations in which the chronic condition develops insidiously, without any preceding acute problem. Type 2 diabetes is an example of this, with immunometabolic dysfunction and chronic systemic inflammation.14
In either case, a complex array of linked biological mechanisms contributes. As illustrated in Figure 1, these mechanisms might include immune system and metabolic dysfunction,14 systemic inflammation,15 oxidative stress,16 dysregulation of the neuroendocrine stress response (hypothalamic–pituitary–adrenal [HPA] axis),17 nervous system sensitisation,9 dysbiosis18 and increased intestinal permeability.19 Specific disease expression in an individual depends upon genetic and epigenetic factors. However, shared biological mechanisms commonly manifest in multimorbidity.
The relative importance of specific mechanisms varies across the spectrum of chronic conditions. The influence of stress with HPA axis dysregulation and chronic systemic inflammation are widely cited as fundamental mechanisms.15 Inflammation is commonly understood to involve peripheral tissues; however, it is now recognised that inflammation can also affect the peripheral or central nervous systems (neuroinflammation). The spillover of systemic inflammation to the nervous system is central to the pathogenesis of chronic pain, anxiety, depression and neurodegenerative disorders.20 The inter-relationships between immune system activation, systemic inflammation and neuroinflammation within the neurovascular unit21 are illustrated in Figure 2. Systemic inflammation can damage the protective glycocalyx22 and the endothelium itself.23 Vascular inflammation can spill over the blood–brain or blood–nerve barrier, contributing to peripheral or central neuroinflammation, with consequent changes in microglia, astrocytes and neurons.21 Metabolic dysfunction, with abnormal carbohydrate metabolism and the inflammatory effect of excess insulin, is important in many chronic conditions.14 Dysbiosis, increased gut permeability and autoimmunity are also strongly associated with chronic conditions.24
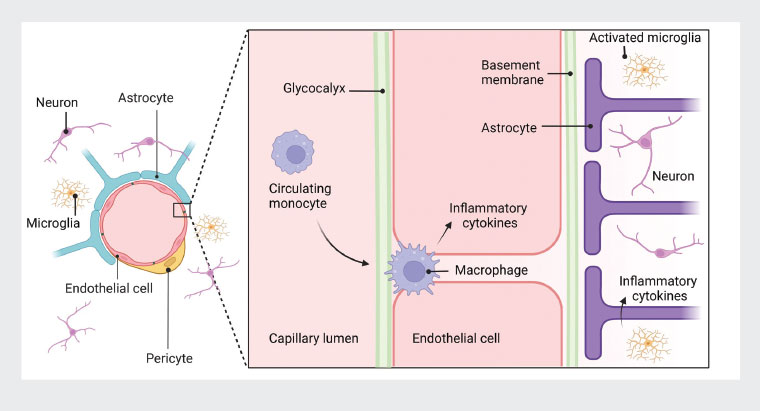 Figure 2. Inflammation within the neurovascular unit. Systemic inflammation might extend from the blood stream (circulating monocytes), through the vascular wall (macrophage infiltration) to the nervous system with activation of microglia and release of inflammatory cytokines.
Figure 2. Inflammation within the neurovascular unit. Systemic inflammation might extend from the blood stream (circulating monocytes), through the vascular wall (macrophage infiltration) to the nervous system with activation of microglia and release of inflammatory cytokines.
Therapeutic possibilities
Challenges in advancing multimorbidity management in primary care have been recognised.25,26 Application of the cumulative strain model might provide a conceptual basis for integrated care. Lifestyle factors, including psychological distress, low or excess physical activity, poor sleep, suboptimal diet, chemical exposure and environmental pollution, have all been implicated in the aetiology of chronic conditions.15 The corollary is that these aspects can be targeted therapeutically to modulate the biology of strain. A detailed exploration of these areas of treatment is beyond the scope of this article. However, brief commentary will be made, predominantly with reference to chronic pain.
Diverse treatments for chronic pain, including psychological and physical therapies, have been shown to bring pain reduction and improved quality of life.1 This is brought about, at least in part, by targeting neuroinflammation and nervous system sensitisation with reversal of maladaptive changes.27 The additional biological mechanisms of strain shown in Figure 1 might also be of relevance (eg targeting the neuroendocrine stress response, again through either psychotherapy or physical activity28). There is emerging evidence that a change in dietary patterns might benefit chronic pain. For example, optimising diet quality and excluding ultra-processed food can address metabolic dysfunction and reduce systemic inflammation, with consequent improvements in pain and mood.29,30
Outcome evaluation from pain services across Australian and New Zealand demonstrates that a behavioural change approach incorporating opioid reduction brings about improvements in pain and function.31 This highlights the broader issue that, when behavioural and lifestyle changes are implemented, it is often possible to reduce reliance on biomedical treatments for chronic conditions.
Conclusion
The proposed cumulative strain model recognises diverse biological mechanisms of strain. It explains why chronic pain often occurs amidst multimorbidity and why unimodal biomedical treatments are often ineffective. It provides hope that lifestyle and behavioural interventions can lessen the biology of strain and benefit chronic pain and multimorbid conditions simultaneously.
The cumulative strain model suggests that reversal of disease processes and recovery of function can begin with the application of lifestyle modulators to target biological mechanisms of strain. A change strategy can be directed towards the individual, the environment or policy aspects. Across the spectrum of chronic conditions, further research is needed to clarify the condition-specific contributions of different biological mechanisms of strain and consequent treatment optimisation.
To achieve greater health system effectiveness, there is a need to evaluate how generic chronic condition treatment programs might be more comprehensively embedded in primary care. In addition, the design of specialist pain treatment programs needs to be adapted to address the issue of multimorbidity. Such changes across the health system have the potential to yield more effective treatment of pain and multimorbid chronic conditions.
Key points
- The cumulative strain model for chronic pain and multimorbidity highlights commonality in underlying mechanisms and treatment.
- A broad approach involving lifestyle and behavioural changes can lessen the biology of strain and benefit people with chronic pain and multimorbid conditions.
- Further development of generic chronic condition programs in primary care might increase efficiency of healthcare delivery and reduce referrals to condition-specific specialist programs.
- Condition-specific treatment programs can benefit from addressing multimorbidity.