COVID-19 is caused by SARS-CoV-2, which primarily targets angiotensin converting enzyme-2 (ACE2) receptors on cells of the respiratory system and induces an immediate immunological cascade of cytokine responses that affect the individual cells and vascular system. Box 1 summarises the pathobiological processes associated with COVID-19 that show strong similarity to the pathophysiological underpinnings of acute ischaemic stroke. Nervous system involvement is common from early in the contagious stage (headaches, dizziness and anosmia)1 to post-infection ‘brain fog’ and fatigue,2–4 with a growing assortment of cases and case series describing a wide array of neurological manifestations generating nearly 2000 PubMed-indexed publications as of 29 November 2020.
| Box 1. Pathobiology of COVID-19 and brain involvement illustrates the shared mechanisms of pathobiology of acute ischaemic stroke and COVID-19* |
The order of the mechanisms described here is not necessarily indicative of the temporal trajectory and potentially may change as the pandemic and long-term neurological effects continue to unfold.
- Binding of SARS-CoV-2 to angiotensin converting enzyme-2 (ACE2) receptor on the surface of alveolar pneumocytes inducing local inflammation.
- Endothelial activation and endotheliitis due to direct viral activity and also via downregulated receptors (ACE2 and other receptors).
- Release of pathogen-associated molecular patterns due to the viral infection and activation of transcription factors such as nuclear factor kappa B, NOD-like receptor protein 3 inflammasome via pattern recognition receptors and ongoing production of cytokines at the entry sites. (Acute stroke induces similar responses following bioenergetic failure secondary to ischaemia, and damage-associated molecular patterns. Similar transcription factors are activated and remain so during the first months as oedema subsides, thus inducing chronic inflammation and suppressing immunological recovery.23,24)
- Prolonged alterations in the balance between the renin-angiotensin-aldosterone system and the downregulated ACE2 pathways are likely to promote end-organ dysfunction in the setting of acute COVID-19.
- Activation of the vascular innate immune system inducing recruitment of macrophages, monocytes and neutrophils to the alveoli.
- Release of tissue factor, Von Willebrand factor, that in turn induces hypercoagulation and prothrombotic state.
- Further recruitment of intravascular neutrophils in the lungs and to microglia in the brain, activates proinflammatory cytokine production.
- Blood–brain barrier disruption.
- Microthrombosis, thromboembolism in the brain.
- The anxiety-induced changes in the hypothalamic–pituitary adrenal system also enhances potential imbalances between sympathetic and parasympathetic activity.
- In the case of vulnerable groups such as those with obesity, hypertension or atherosclerosis, and vulnerable older groups with already compromised vascular systems and maladaptive immune responses, a post-COVID-19 hyperimmune response is an expectation rather than the exception.
|
*Many of these processes are well described in acute ischaemic stroke and long-term effects of stroke
among survivors. The authors of this editorial are currently studying both groups and observed the strong similarities between the two entities. |
The COVID-19-associated neurological disorders reported to date fit into five main categories: encephalopathies, inflammatory central nervous system (CNS) syndromes, ischaemic strokes, peripheral neurological disorders and miscellaneous other CNS disorders.5 The number of articles reporting longer-term post-COVID-19 neurological effects and symptoms makes an undeniable case for medicine to recognise the Post-COVID-19 Neurological Syndrome (PCNS)3,6–9 and the need for ongoing monitoring of all cases of COVID-19 for post-syndrome neuropsychiatric symptoms, irrespective of the clinical severity of the acute infection. The extent of post-syndrome effects has not yet been rigorously researched, but studies such as Goertz et al – based on anecdotal social media reports of approximately 2000 patients in Belgium and the Netherlands who had previously experienced mild-to-asymptomatic COVID-19 – suggest symptoms continue for months, highlighting the need for ongoing vigilance for PCNS symptoms by general practitioners (GPs).3 Kingston et al from the UK have also documented persistent symptoms following COVID-19 infections through qualitative analysis of 24 interviews, with repeated mention of ‘brain fog’ and a myriad of symptoms among many of them.1 PCNS is an evolving story, and we are currently studying a series of patients with potential PCNS as we pen this editorial.
The first Australian case of acute COVID-19 with severe neurological involvement10 has demonstrated the association of rapid elevation in the immunological markers neutrophil/lymphocyte ratio11–14 and raised inflammatory biomarkers such as C-reactive protein and D-dimer, and correlation with disease severity (Figure 1).1
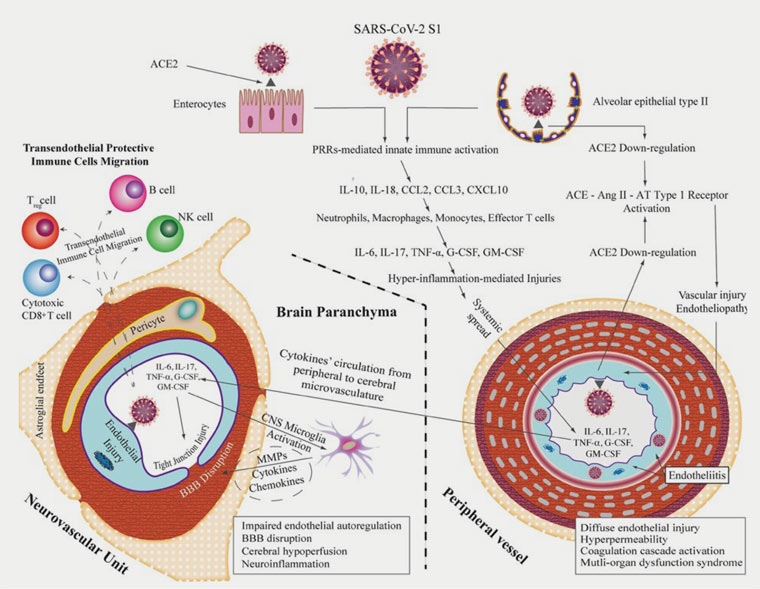
Figure 1. Integrative concepts of the pathophysiology of COVID-19-related central nervous system complications. This figure integrates the clinical and experimental data linking maladaptive innate immunity-related systemic hyperinflammation (provoked by the binding of SARS-CoV-2 S1 to ACE2-expressing cells in the lung and intestine) to neurovascular endothelial dysfunction, BBB breakdown and CNS innate immune activation, potentially contributing to SARS-CoV-2-related CNS complications. It demonstrates subsequent endothelial injury in the peripheral vasculature due to direct viral endothelial infection causing endotheliitis and potential endothelial ACE2 downregulation; similar mechanisms may also involve the neurovascular unit. It also depicts the proposed role of infiltrating protective immune cells, migrating from the bloodstream into the CNS parenchyma through the disrupted BBB, in limiting CNS injury and promoting viral clearance.25 Click here to enlarge
ACE, angiotensin converting enzyme; ACE2, angiotensin converting enzyme 2; Ang II, angiotensin II; AT, angiotensin; BBB, blood–brain barrier; CCL, CC chemokine ligand; CNS, central nervous system; CXCL, chemokine (C-X-C motif) ligand; G-CSF, granulocyte colony stimulating factor; GM-CSF, granulocyte-macrophage colony stimulating factor; IL, interleukin; MAP, microglial activation and proliferation; MMPs, matrix metalloproteinases; NK, natural killer; PRRs, pattern recognition receptors; SARS-CoV-2 S1, severe acute respiratory syndrome coronavirus 2 spike glycoprotein 1; TNFα, tumour necrosis factor-α
Reproduced from Najjar S, Najjar A, Chong DJ, et al, Central nervous system complications associated with SARS-CoV-2 infection: Integrative concepts of pathophysiology and case reports, J Neuroinflammation 2020;17(1):231, doi: 10.1186/s12974-020-01896-0, licensed under CC BY-NC 4.0.
Similar pro-inflammatory immunological responses to acute SARS-CoV-2 infection in some cases result in severe immune overreaction with excessive neutrophil activation and cytokine release syndrome (‘cytokine storm’) and alteration in coagulation, consequently leading to neurovascular thrombosis and ischaemia as have been described in a number of cases worldwide.15,16
Infection-driven activation of the extrinsic coagulation system and decreased nitrous oxide levels have been hypothesised as underpinning the role of the vascular endothelium in the pathobiology of COVID-19. Moreover, the renin-angiotensin system and ACE2 deficiency have also been postulated to play a critical part in worsening the prognosis of COVID-19-related neurovascular involvement among patients with diabetes and hypertension.
Post-COVID-19 Neurological Syndrome
The build-up of pro-inflammatory agents such as interferon-gamma, interleukin-7 and other cytokines is known to promote and propagate post-stroke depression, which shows significant similarity to the pathobiology of COVID-19.17–19 As alluded to previously, many potential cases of PCNS are already well reported in social media posts as well as more rigorously described in scientific published articles.8,9,20,21 While the full clinical picture of PCNS, disease mechanisms and potential treatment will be studied and revealed in the coming months, The Royal Australian College of General Practitioners’ (RACGP’s) publication Caring for adult patients with post‑COVID‑19 conditions22 provides useful information and advice.
The RACGP publication highlights six common post-acute COVID-19 symptoms as follows:
- fatigue
- dyspnoea
- joint pain
- chest pain
- cough
- change in sense of smell or taste.
The publication lists the less common symptoms as insomnia, low-grade fevers, headaches, neurocognitive difficulties, myalgia and weakness, gastrointestinal symptoms, skin rash and depression. The publication also provides a useful approach to management of the common symptoms that includes helpful tips on management of fatigue, neurocognitive difficulty and anxiety.
Conclusion
The pandemic statistics highlight the need for ongoing, careful follow-up of all patients with COVID-19, even those formerly regarded as asymptomatic, with regular screening for likely long-term persistent neurological involvements. Such cases also call for rapid communication lines between GPs and neurologists through direct email or telephone communications through neurology registrars at least once to correctly document and study these cases. The more complete nature of PCNS and its myriad of clinical features will unfold in the coming months. Many local and global research groups are currently working in these areas, and several Medical Research Future Fund grants will be available to run the necessary clinical trials in the coming months. It is necessary for GPs in Australia to be aware of the long-term effects and continue to monitor patients with previous COVID-19 infection when the opportunity arises, as GPs are the likely first point of contact. The rapidly evolving knowledge regarding the virus, the effects of acute infection and the resulting long-term effects of chronic inflammation will require continuous education and access to any potential interventional clinical trials aimed at ameliorating the long-term neuropsychiatric problems associated with SARS-CoV-2 infection.
First published online 12 January 2021.