More than 270,000 Australians receive residential aged care annually,1 and 10–20% of residents have diagnosed type 2 diabetes mellitus (T2DM).2 Residents are often at high risk of adverse drug events (ADEs) because of multimorbidity, dementia, complex medication regimens, polypharmacy and renal impairment.1,3–7 Optimising medication management of T2DM is important because five of the 15 most implicated medications in emergency department presentations are glucose-lowering medications (GLMs).8 Compared with people aged 45–64 years, those aged ≥80 years are more than twice as likely to visit an emergency department for insulin-related errors and five times more likely to be subsequently hospitalised.9 Meal-planning misadventures (eg not eating shortly after administration of rapid-acting insulin or not adjusting insulin regimen for reduced food intake) and insulin product mix-ups are leading causes of insulin-related hypoglycaemic events.9 A small cross-sectional study undertaken in 10 Victorian residential aged care facilities (RACFs) showed seven (6.5%) of the 108 residents with diabetes had unplanned diabetes-related hospitalisations over a six-month period.2 Optimal management of T2DM in RACFs requires consideration of evidence-based guidelines.
This article builds on the foundation provided by the current Royal Australian College of General Practitioners’ (RACGP’s) clinical management guidelines for T2DM10 and highlights practical details for clinicians providing care for residents. Because there is limited controlled trial evidence from residential aged care, existing guidelines for the management of T2DM in this setting are largely based on extrapolation of evidence from other settings, expert opinion and/or observational studies. While the focus of this article is on GLMs, clinicians should also be aware of the importance of optimising antihypertensive and lipid-lowering medications in residents with T2DM.10
Principles of optimal T2DM management in RACFs
Management of T2DM in RACFs typically has less emphasis on glycaemic control and greater focus on maintaining residents’ quality of life. Intensive glycaemic control may increase the risk of adverse events and mortality, and only reduces microvascular rather than macrovascular complications.11,12 Conversely, optimal glycaemic management may help to prevent acute symptoms of T2DM (eg polyuria, weight loss, fatigue, confusion and falls).
Australian guidelines (ie The McKellar guidelines for managing older people with diabetes in residential and other care settings and Diabetes Australia’s Diabetes management in aged care: A practical handbook) recommend individualised glycated haemoglobin (HbA1c) targets of 53–64 mmol/mol (7–8%) for most residents.3,11 Target HbA1c levels of 53–59 mmol/mol (7–7.5%) are recommended for functionally independent residents, and targets of up to 69 mmol/mol (8.5%) may be suitable for frail residents and residents living with dementia.3,11 Australian RACF guidelines also recommend six‑monthly HbA1c monitoring if glycaemic management is stable, and three-monthly monitoring if management is suboptimal.3 Recent international guidelines recommend against targeting HbA1c levels and instead treating residents only to minimise hyperglycaemia-related symptoms.12 Nevertheless, all accepted guidelines point to individualised care,3,11,12 and we suggest that care is provided across a continuum ranging from residents with long remaining life expectancy and minimal functional and/or cognitive impairment whose T2DM care resembles standard care through to those with limited life expectancy and severe functional/cognitive impairment treated to minimise symptoms (Figure 1).
When hyper/hypoglycaemic symptoms are present, or if there is a change in management, Australian RACF guidelines recommend that blood glucose levels (BGLs) are measured three to six times per day (ie pre-meals and two hours post-meals) initially.3,11 Once stable, and depending on the resident’s condition, less frequent pre-meal and post-meal BGL monitoring (eg once per day to once every three days) may be appropriate;11,13 however, monitoring of BGLs is no longer routinely indicated for residents who are not receiving insulin and have stable BGLs.14 Occasional testing between 2.00 am and 3.00 am could be considered if nocturnal hypoglycaemia is suspected.11 Australian RACF guidelines advise monitoring BGLs in the setting of acute illness or weight loss, using an individualised BGL target range of 6–15 mmol/L.3,11
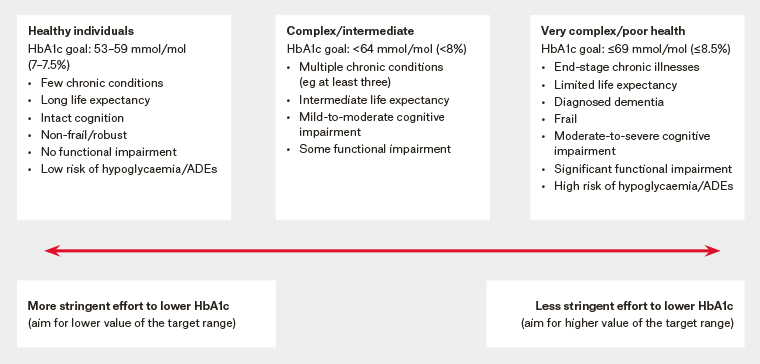
Figure 1. Consensus framework for individualising targets and therapeutic approach to glycaemic management across the continuum of care for older people with type 2 diabetes mellitus3,10,11,34
ADEs, adverse drug events; HbA1c, glycated haemoglobin
Pharmacological treatment options for T2DM management in RACFs
Medication management considerations relevant to care planning by general practitioners (GPs) are summarised in Box 1. When assessing medication management for a resident with T2DM, guidelines suggest that the resident, family members, aged care provider staff, GPs and pharmacists should be familiar with the benefits and risks of treatment in the context of the resident’s goals of care.
3,11,13
|
Box 1. Medication-related care plan considerations for residents with type 2 diabetes mellitus3,11,13,16–18,21,35–37
|
-
Carefully evaluate resident comorbidities, overall health and resident/carer preferences.
-
Ensure a sensitive discussion and documentation of an individualised treatment plan, glycaemic targets and strategies for medication management.
-
Start low and go slow with doses when initiating and/or changing medications.
-
Assess and minimise the risk of hypoglycaemia and other ADEs related to GLMs. Consider use of the following resources when assessing medication use:
-
GLM-related ADEs risk assessment tool (available from the McKellar guidelines for managing older people with diabetes in residential and other care settings)3
-
Beers criteria for potentially inappropriate medication use in older adults17
-
STOPP: screening tool of older people’s potentially inappropriate prescriptions, and START: screening tool to alert doctors to right treatments35
-
Medication appropriateness index36
-
Australian inappropriate medication use and prescribing tool37
-
Australian Medicines Handbook Aged Care Companion18
-
A new blood glucose management algorithm for type 2 diabetes: A position statement of the Australian Diabetes Society21
-
Consider use of non-pharmacological alternatives where possible.
-
Simplify treatment regimens.
-
Avoid sliding scale insulin.
-
Conduct annual testing of eGFR (by a blood test) and urine ACR by a urine test for screening and monitoring of CKD, for residents who are otherwise ‘healthy’ and whose care resembles standard care.
-
Seek multidisciplinary input (eg from diabetes educators, aged care staff, pharmacists, allied health) where necessary.
-
Consider reviewing management when falls, confusion and non-specific ‘incidents’ occur.
-
Ensure the resident, family members and aged care provider staff are educated regarding resident self-monitoring, documentation of BGLs, symptoms of hypoglycaemia and hyperglycaemia, and sick day medication management strategies. A comprehensive approach to sick day management is available from current Australian RACF guidelines.3,11
|
|
ACR, albumin/creatinine ratio; ADEs, adverse drug events; BGLs, blood glucose levels; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; GLMs, glucose lowering medications; RACF, residential aged care facilities
|
Important considerations when selecting and monitoring GLMs
Residents’ age, functional and cognitive status, acute illness, comorbidities and past medical history, renal function, frailty and hypoglycaemia risk are important to consider when selecting and monitoring GLMs for those with T2DM.13,15 Hypoglycaemia risk is one of the most important determinants of medication choice and glycaemic monitoring.13,15 Metformin is generally considered first-line because of its established safety and efficacy.3,13 However, nearly half of all residents have clinical evidence of chronic kidney disease.7 Annual renal function monitoring is recommended by guidelines but is often overlooked.11,16 Guidelines also recommend renal checks every three to six months if there is evidence of microalbuminuria or proteinuria.11 Several GLMs should be prescribed with caution or may not be appropriate in residents with worsening renal function and those aged ≥75 years (Table 1).17–21 The presentation of ADEs in older people may be non-specific and more challenging to recognise,22 particularly among residents living with dementia who may not be able to readily communicate their symptoms.
|
Table 1. Glucose-lowering medications used in type 2 diabetes mellitus management in residential aged care facilities3,11,13,17-21
|
|
Medication
name/class
|
Hypoglycaemia risk
|
Dosage reduction required for renal impairment
|
Contraindicated in renal impairment
|
Other considerations relevant to selection, monitoring or de‑intensification
|
|
Metformin
|
Minimal
|
Yes
CrCL 60–90 mL/min, maximum 2 g daily
CrCL 30–60 mL/min, maximum 1 g daily
CrCL 15–30 mL/min, maximum 500 mg daily, but use with caution
|
Yes, when CrCL <15 mL/min
|
-
May cause weight loss and GI upset
-
Cease if diarrhoea continues for a few days after starting, even after dose reduction
-
Extended-release form has fewer GI side effects and may reduce regimen complexity
-
In renal impairment, cease if at risk of further decline in renal function.
|
|
Sulfonylureas
(eg glibenclamide, gliclazide, glimepiride, glipizide)
|
High
|
Reduce dose of glibenclamide, gliclazide, glipizide in severe renal impairment
Glimepiride
CrCL >30–50 mL/min, maximum 1 mg daily
|
Yes, (glimepiride) when CrCL <30 mL/min
Yes, (glipizide) in renal insufficiency
|
-
Efficacy may reduce over time as beta cell function is gradually lost in T2DM
-
Long-acting sulfonylureas (glimepiride, glibenclimide and slow-release gliclazide) have a higher risk of hypoglycaemia and should generally be avoided in frail residents and when eating patterns are irregular.
|
|
DPP-4 inhibitors
(eg alogliptin, linagliptin, saxagliptin, sitagliptin, vildagliptin)
|
Low
|
Alogliptin
CrCL 30–50 mL/min, maximum 12.5mg daily
CrCL <30 mL/min, maximum 6.25 mg daily
Sitagliptin
CrCL 30–50 mL/min, maximum 50 mg daily
CrCL <30 mL/min, maximum 25 mg daily
Saxagliptin
CrCL <50 mL/min, maximum 2.5 mg daily
Vildagliptin
CrCL <60 mL/min, maximum 50 mg daily
|
|
-
All are given once daily, except vildagliptin (given once or twice daily)
-
All require dose reduction in renal impairment, except linagliptin (excreted unchanged in bile).
|
|
GLP-1 receptor agonists
(eg exenatide, liraglutide)
|
Low
|
Exenatide
CrCL 30–50 mL/min, dose escalation from 5 to 10 microgram should proceed conservatively
No dose adjustment required for liraglutide if CrCL ≥30 mL/min
|
Yes, (exenatide) when CrCL <30 mL/min
|
-
May cause weight loss. Avoid in residents who are frail and underweight
-
GI effects more common in elderly
-
Liraglutide initiation is not recommended in people aged ≥75 years and in end-stage renal disease (no experience in these groups).
|
|
Acarbose
|
Low
|
No
|
Yes, when CrCL <25 mL/min
|
|
|
Thiazolidinediones
(eg pioglitazone, rosiglitazone)
|
Low
|
No
|
|
-
May worsen heart failure and increase risk of bone fracture
-
Change in glycaemic control may take up to 12 weeks after initiation, dose changes or cessation.
|
|
SGLT2 inhibitors
(eg dapagliflozin, empagliflozin)
|
Low
|
No
|
Yes (dapagliflozin) when CrCL <60 mL/min
Yes (empagliflozin) when CrCL <45 mL/min
|
-
Watch for increased urinary frequency/incontinence, genitourinary infections and dehydration, which can lead to delirium
-
Not recommended with loop diuretics due volume depletion concerns
-
May be problematic in residents with urinary incontinence and those who require assistance getting to the toilet
-
SGLT2 inhibitors initiation is not recommended in people aged ≥75 years and in end-stage renal disease (no experience in these groups).
|
|
Insulin
|
High
|
Yes; requirements for all insulins may be reduced with renal impairment because of altered insulin metabolism.
|
|
-
Appropriate meal planning is essential
-
Basal insulin may have lower hypoglycaemia risk than premixed insulin
-
Insulin administered via a syringe poses an increased risk of overdose. Administration via a pen device is preferred in RACFs.
|
|
CrCL, creatinine clearance; DPP-4, dipeptidyl peptidase-4; GI, gastrointestinal; GLP-1, glucagon-like peptide-1; RACFs, residential aged care facilities; T2DM, type 2 diabetes mellitus; SGLT2, sodium-glucose co-transporter 2; DPP-4 inhibitors, dipeptidyl peptidase-4 inhibitors; GLP-1, glucagon-like peptide-1 receptor
|
Table 1 presents special considerations and practical hints for clinicians managing GLMs in the RACF setting. If multiple oral GLMs are required, fixed-dose combination products may assist with medication regimen simplification, although initiation of single-ingredient medications prior to combination products is recommended.20
Before initiating insulin or adjusting insulin doses, it is important to exclude factors that may have precipitated hyperglycaemia (eg urinary tract infections, high-dose thiazide, corticosteroids)13,20 and assess factors such as cognitive function that may have an impact on the resident’s ability to recognise and report symptoms of hypoglycaemia. Insulin glargine is a long-acting basal insulin often prescribed to residents that is usually administered once daily. It may be withheld if a resident’s fasting/pre-meal BGL is considered low, although it is important that nursing staff understand the reasons behind this approach.3,11,13 We suggest that nurses and care staff are made aware of what BGL is considered low for an individual, particularly given the variation in the definition of acceptable BGL ranges in current RACF guidelines (eg values at the lower end of the acceptable BGL range for a resident with T2DM commence at 5.5 or 6 mmol/L in current guidelines).3,11,13 Sliding-scale insulin is complex to administer. It has a high risk of hypoglycaemia without improving hyperglycaemia management.13,17 Alternatively, basal insulin combined with oral GLMs may lower postprandial BGLs while reducing hypoglycaemia risk and medication regimen complexity.13
Important considerations for de-intensification of GLMs
Factors that may affect the decision to de-intensify GLMs include terminal illness, renal impairment, stable or non-existent glycaemic symptoms, complex medication regimens, polypharmacy, intolerable ADEs, and resident or family preferences.13,23,24 Current guidelines recommend de-intensification or cessation of GLMs in frail residents living with advanced dementia and residents with limited life expectancy, but there is limited evidence regarding the optimal approach.23,24 Box 2 offers practical tips for clinicians when de-intensifying medication use in RACFs. Stopping insulin in residents living with type 1 diabetes is not appropriate and can result in disastrous clinical outcomes (eg metabolic decompensation and ketoacidosis).25
|
Box 2. Tips for de-intensification of medications among residents with type 2 diabetes mellitus3,23,24
|
-
Identify residents with T2DM who may benefit from de-intensification of prescribed GLMs or other medications that may be affecting BGLs.
-
Have a non-judgemental discussion with the resident (and family members as appropriate) to discuss the reasons for each medication and the reasons for any medication refusals.
-
Once assessed, discuss potential benefit and harm associated with prescribed medications.
-
Prioritise with the resident those medications that the resident is most willing and comfortable to discontinue and those with the greatest harm and least benefit.
-
In partnership with the resident (and family members as appropriate), negotiate and implement a de-intensification regimen that incorporates:
-
reducing dose or ceasing medications that are most likely to cause hypoglycaemia; or
-
switching to a different agent with lower hypoglycaemic risk if possible; or
-
reducing dose of renally eliminated GLMs (eg metformin, exenatide, dapagliflozin, empagliflozin, insulin, glibenclamide, gliclazide, glimepiride, glipizide, alogliptin, saxagliptin, sitagliptin) using strategies such as:
-
ceasing or weaning off one medication at a time to confirm the benefits (improved outcomes or reduced adverse effects) and harm (hyperglycaemia) due to de-intensification, and then reassess
-
requesting that aged care provider staff closely monitor the resident (daily for 1–2 weeks) after each medication change for signs of hyperglycaemia and hypoglycaemia, and/or resolution of the ADEs, and document observations.
-
Communicate the plan to health professionals, aged care provider staff and family involved in resident’s care.
-
Unless the situation is urgent (eg presence of hypoglycaemia symptoms or acute illness), assess BGL within 24–48 hours after a change in management.
-
If symptomatic hyperglycaemia results following de-prescribing of GLMs, restart the medication or consider an alternative GLM with lower risk of hypoglycaemia.
|
|
ADEs, adverse drug events; BGL, blood glucose level; GLMs, glucose-lowering medications; RACF, residential aged care facilities; T2DM, type 2 diabetes mellitus
|
End-of-life care for residents with T2DM
Guidelines for the management of T2DM at the end of life are primarily based on expert opinion, surveys and retrospective studies.26
When a resident has a prognosis of weeks to months, a recommended approach is a sensitive explanation and reassurance about relaxing glycaemic management for all residents with T2DM who have previously been used to an emphasis on intensive glycaemic management.27 As oral food intake declines, glycaemic management can be relaxed and dietary restrictions can be removed.26 When a resident with T2DM has a prognosis of weeks to months, insulin can often be ceased if the prescribed dose is small.27 Clinical judgement is key, because there is no clear consensus in the literature on what dose is considered small and which insulin types could be ceased, and expert opinions vary.
In the last weeks of life, routine BGL monitoring is not advised; however, minimal testing may be necessary when medication doses are changed and to avoid unpleasant acute hypoglycaemic and hyperglycaemic symptoms.10,27–29 Oral agents and glucagon-like peptide-1 (GLP-1) agonists can also be ceased.27 For residents with T2DM in the last weeks of life who are receiving insulin, it may be prudent to only cease insulin if the dose is small.27 Otherwise, changing to a once-daily morning dose of isophane insulin or insulin glargine that is equivalent to 75% of the total previous daily insulin dose is suggested. The BGL can be checked once daily at dinner time, with insulin doses altered by 10–20% if the BGL is outside an 8–20 mmol/L target.27 If BGL is >20 mmol/L after insulin is ceased, which may occur if corticosteroids are used for ongoing symptom management, then short-acting insulin may be given to reduce symptom burden.27,29,30 If insulin is required more than twice, consider prescribing insulin isophane or glargine daily.27
In the final days of life, residents may have reduced consciousness but still may be able to take sips of fluids. At this time, the goal of care is to minimise symptomatic hypoglycaemia or hyperglycaemia and reduce the burden of invasive testing and treatments.29
Strategies and tools for optimising medication management in RACFs
Optimisation of GLMs often necessitates collaboration with health professionals such as nurses, aged care staff, pharmacists, dietitians and diabetes educators for input into optimising insulin use, optimal nutrition and meal planning, and ensuring education for nurses and care staff regarding T2DM management.3,11,13,15 The following strategies and tools may help to manage the complex processes that underpin prescription, supply, administration and monitoring of GLMs in RACFs:
-
Residents with T2DM often have complex medication regimens.31 The Medication Regimen Simplification Guide for Residential Aged CarE (MRS GRACE) is a five-step guide validated by pharmacists that GPs, nurse practitioners or pharmacists can use to identify opportunities to simplify medication regimens in RACFs.4 It prompts the user to consider the resident, regulatory and safety requirements, drug interactions, medication formulations, and facility and follow-up considerations when identifying opportunities to reduce unnecessary medication complexity.4
-
Residents with T2DM can be referred for a residential medication management review (RMMR). This collaborative service involves a pharmacist accredited to undertake RMMRs visiting the RACF to conduct a comprehensive medication review involving the resident, family members and/or aged care provider staff.32 The pharmacist then develops a suggested medication management plan and communicates the recommendations to the GP and aged care provider staff.32
-
Medication Advisory Committees play an important role in monitoring the use of high-risk medications and ensuring RACF staff are sufficiently trained to monitor BGLs, administer GLMs, and recognise and respond to ADEs, incidents and near misses.3,11,33
-
The national residential medication chart supports consistency and includes safety features such as a section for insulin prescribing and administration.1
Conclusion
Medication management of T2DM in RACFs requires consideration of resident-specific factors such as multimorbidity, cognitive decline, frailty, complex medication regimens, polypharmacy, renal function, vulnerability to ADEs and end-of-life care needs. Intensive management of glycaemic control is no longer recommended in RACFs. Optimal T2DM management remains an important challenge for residents, aged care providers and health professionals. Nevertheless, a multidisciplinary team approach centred on individualised resident goals of care is essential to improving T2DM management, reducing risk of ADEs and optimising resident health outcomes and quality of life.