Obstructive sleep apnea (OSA) is a condition characterised by partial or complete periodic obstruction of the upper airway during sleep. It affects up to 9% of women and 24% of men in Western society,1 although more recent studies suggest the prevalence of OSA in the general population is considerably higher.2 This is often coupled with snoring, oxyhaemoglobin desaturation, repeated arousals and fragmented sleep.2 This review provides a clinical update on the recent developments and future directions in the individualised management of OSA.
Complications of untreated OSA
Untreated OSA can cause significant short-term complications, such as impaired neurocognitive function and daytime somnolence.3 In the longer term, OSA has been linked with systemic hypertension, myocardial infarction, congestive heart failure and stroke, contributing to the burden of chronic diseases and resulting in significant economic cost to the healthcare system.4,5
Aetiology
The aetiology of OSA is multifactorial, making effective management of the condition challenging. However, a comprehensive understanding of individual phenotypic characteristics that contribute to OSA can allow for targeted, effective multimodal treatment. The history should include factors relating to the diagnosis of OSA (snoring, witnessed pauses or gasps, positional differences, waking from apnoea), the consequences of OSA (daytime somnolence, waking unrefreshed, poor cognitive function, cardiometabolic comorbidities) and the existence of concomitant sleep disorders (insomnia, sleep architecture). Standardised questionnaires such as the OSA50, STOP-BANG and Berlin can be used as screening tools for OSA diagnosis; however, they have been found to be low in specificity.6 Other questionnaires used before and after treatment to determine therapeutic effect include the Epworth Sleepiness Score (ESS), Snoring Severity Score, FOSQ-10 and FOSQ‑30.7
General examination and visualisation of the airway allow assessment of potential contributing anatomy. Blood pressure and body mass index (BMI) are important to note, particularly as obesity is a significant contributing factor.8 Further examination includes craniofacial structure, jaw position (eg retrognathia), neck and abdominal circumference, the oral cavity and oropharynx. Important transoral features to note include a high arched narrow palate, elongated uvula, malocclusion, tongue and tonsil size (incorporating the modified Friedman staging system).9,10
Dynamic airway assessment via flexible nasendoscopy is performed to determine sites and planes of airway collapse, which may include palatine and lingual tonsil hypertrophy, retrolingual and retropalatal collapse and, rarely, laryngeal pathology.11 Examination of the nasal airway reveals factors that may affect continuous positive airway pressure (CPAP) or mandibular advancement splint (MAS) compliance including septal deviations, septal spurs, nasal polyps and hypertrophic inferior turbinates.12
Diagnosis
Recent changes in the Australian Medicare Benefits Schedule (MBS) mandate that direct referral for polysomnography via a general or other practitioner is only permitted if questionnaire screening (OSA50 >5 or STOP-BANG >4, and ESS >8) criteria are met. Otherwise, referral to a sleep physician for assessment prior to a polysomnogram is required.13
For many years, the standard for diagnosis of OSA has been formal in-laboratory polysomnography (PSG). However, there is a progressive shift toward limited and full-channel sleep studies that can be performed at home.3 Limited channel home sleep studies have good specificity in uncomplicated patients with a high pre-test probability of moderate-to-severe OSA.14 However, these studies become less reliable in patients who have a number of comorbidities and in the presence of comorbid sleep disorders.15
Management of OSA
Individualised patient goals and motivations should be taken into consideration. This often includes symptom control, with the usual foci being reduction in snoring and improvement in general wellbeing including tiredness and daytime sleepiness. The treating physician should also aim for reduction in cardiovascular risk factors and seek improvement in polysomnographic indices such as Apnoea–Hypopnea Index (AHI) and nocturnal oxygen desaturation.3 For example, in a patient with hypertension the use of CPAP to prevent long-term cardiovascular sequalae can be efficacious.16 Contrarily, a recent large randomised control trial found that treatment with CPAP may not reduce risk of further cardiovascular events in those patients with OSA who have already had an infarct.17 Clinical effectiveness is dependent on adequate compliance, generally considered to be >4 hours per night.18 Furthermore, the effect on reduction in blood pressure is more likely to be seen in those patients with baseline hypertension, resistant hypertension and severe OSA.19–21 Therefore, with an individualised focus, the aim should be to gain optimal impact of treatment with maximal compliance.
OSA is a chronic condition that requires long-term follow-up and may require multidisciplinary team input.22 The role of the general practitioner (GP) is paramount in diagnosing a sleep-related disorder with focused history, examination and PSG, as well as coordinating care and adjusting modifiable lifestyle factors.
Sleep physicians play a crucial part in diagnosing and managing OSA with CPAP or other therapeutic options. In patients requiring multimodal therapy, referral to specific sleep multidisciplinary team meetings – which incorporate nurse practitioners, sleep psychologists, exercise physiologists, physiotherapists, orthodontists, sleep physicians and otolaryngologists – plays an important part in ensuring all aspects contributing to patients’ disorders are optimised to gain maximal treatment benefit.
Adjuncts to OSA management
Medical and surgical weight loss options should be considered in conjunction with dieticians, exercise physiologists, physiotherapists and endocrinologists.3 A reduction in BMI has been associated with improvement of OSA; however, few patients achieve complete normalisation of parameters.23 Cardiovascular exercise has been shown in a recent meta-analysis to reduce OSA parameters including AHI.24 Bariatric surgery is a consideration in patients with morbid obesity and comorbidities.25
Device methods of OSA management
Positive pressure treatments
CPAP is the most efficacious therapy to treat OSA (Figure 1).24 Neurocognitive benefits noticed by patients may not always closely correlate with improvements detected during PSG.26 Despite this, a linear improvement in both subjective and objective daytime sleepiness in patients with severe OSA has been found when CPAP is used up to seven hours per night.27
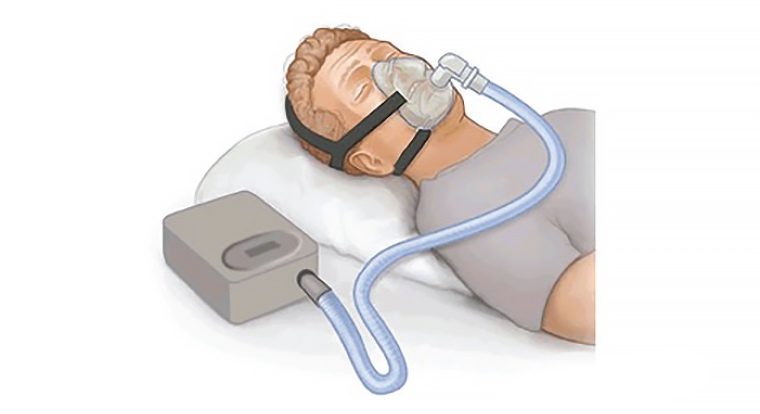
Figure 1. A CPAP device fitted to provide positive pressure to nasal and oral cavities during sleep.
‘CPAP.png’ by PruebasBMA is licenced under CC BY-SA 3.0. The original image can be found at https://commons.wikimedia.org/wiki/File:CPAP.png [Accessed 21 February 2019].
CPAP is limited by compliance, with many patients using the device for an inadequate period of time or refusing to use the device outright.28 This is a multifactorial problem, including disease-specific and device-related factors, as well as patient and psychosocial factors. Common factors include patient and/or partner intolerance of the device, unmet patient expectations, suboptimal effect on symptoms and side effects (including bloating, numbness and CPAP-induced rhinitis).3
Advances in devices allowing automatic titration pressures permit greater individualisation of therapy and, therefore, comfort. New technology via web-based applications and cloud-based software allows patients and physicians to engage with therapy by self-monitoring of adherence and response to therapy. Advances in mask technology have tended to improve mask fit and comfort. To sustain compliance, regular follow-up is required to address issues promptly and effectively.
Mandibular advancement splints
MAS therapy results in a prognathic mandibular position to enlarge and stabilise the airway to minimise airway collapse and soft tissue fluttering (Figure 2). While usually less efficacious than CPAP at reducing AHI, MAS therapy has a role in treating patients with mild-to-moderate OSA and can be effective in carefully selected patients with severe OSA. Splints have been shown to reduce AHI, daytime somnolence and hypertension.16,29,30 However, the results are considerably more variable when compared to CPAP.31 Nevertheless, the health outcomes associated with MAS treatment are similar to those achieved with CPAP, reflecting the superior compliance associated with MAS therapy.
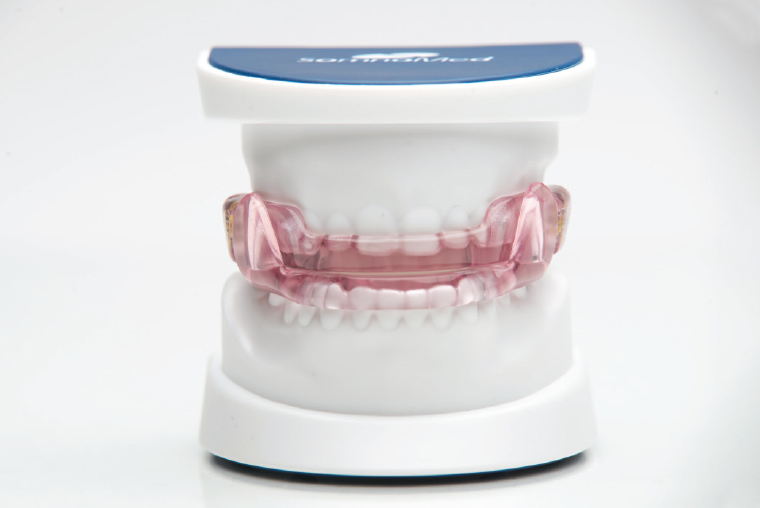
Figure 2. A mandibular advancement splint fitted to upper and lower dentition used to protrude the mandible forward during sleep.
Image courtesy of Somnomed.
Patients for whom MAS therapy is most effective is unclear. A comprehensive review revealed that complete response to treatment was obtained in 48% of patients.29 Factors such as lower AHI, younger age, female gender and lower BMI have been associated with better outcomes.29,32 Other factors, including dynamic airway assessment using nasendoscopy and site of pharyngeal collapse, have shown variable accuracy and clinical applicability without validation in subsequent samples.31
Potential side effects and complications include temporomandibular joint pain, dental pain and movement of dentition. To minimise such issues and improve compliance, appropriate fitting, subsequent titration and long-term follow-up should be performed by a credentialed dentist.
Positional therapy and other devices
In over half of patients with OSA, respiratory events are most frequent and severe in the supine position.33 Prevention of supine positioning during sleep in this subgroup of patients is an effective but variably tolerated method to treat OSA. Although the improvement in AHI is not as pronounced as in patients on CPAP, positional therapy is useful as an adjunct. Positional devices include vibratory (Nightshift and Buzzpod; Figure 3) and non-vibratory (Zzoma and Rematee) devices.
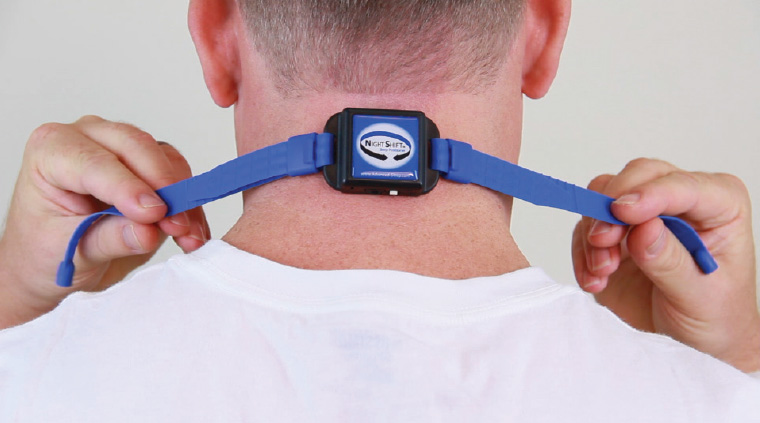
Figure 3. A vibratory sleep device placed around the neck which vibrates when patient is in supine position. Vibration will stop when patient rolls out of supine position.
Image courtesy of Nightshift.
Nasal expiratory positive airway pressure devices sit on the inside of the nasal vestibule, splinting the airway open in expiration by providing resistance. While these devices have been shown to improve disease parameters, results are not as pronounced as with CPAP, and tolerance levels are generally poor.34
Surgical methods of OSA management
The two main roles for surgery are to increase compliance with devices (CPAP or MAS) or to reduce the severity of OSA and its symptoms.35 In some cases, surgery may be performed earlier or as a first-line treatment.35
Nasal surgery
In general, nasal obstruction is addressed first if present. Nasal surgery aims to improve patency by focusing on correction of a deviated septum, reduction of enlarged inferior turbinates, adenoidectomy (if excessive adenoid tissue is present) and stabilisation of the lateral alar cartilage if dynamic collapse is present on inspiration.35 A recent meta-analysis revealed that nasal surgery in patients with OSA and nasal obstruction increased CPAP device use and reduced CPAP pressure.36
Surgery for the palate
While tonsillectomy in isolation has proven effective in treating patients with enlarged tonsils and mild-to-moderate OSA, the procedure is often performed in conjunction with a modern variant of uvulopalatopharyngoplasty (mUPPP).35,37,38 The mUPPP aims to broaden the pharyngeal airway in patients who have soft palate collapse against the posterior pharyngeal wall during sleep. Randomised trials have shown significant improvement in AHI, daytime sleepiness and snoring, with up to 65% of patients requiring no further treatment.39,40 Transpalatal advancement pharyngoplasty can also be performed to provide further anterior re-positioning of the soft palate when required.41
Tongue surgery
When tongue size and position are identified as contributing factors, tongue surgery may be beneficial. This can be achieved effectively via radiofrequency,42 coblation,38 midline glossectomy and other variants,43,44 lingual tonsil reduction45 or, rarely, tongue tensing surgery.46 Trans-oral robotic surgery has been used with varying success.47
Hard tissue surgery
Maxillo-mandibular advancement (bi-max) surgery is a proven, effective global airway procedure.48 It is performed either as ‘phase II surgery’ after the above interventions or, in cases of severe skeletal deficiency, earlier in treatment paradigms.48
Complications of surgery
The most frequent issue of surgical intervention is pain that peaks between days four and seven but often lasts two weeks post-surgery. This may result in limited oral intake and weight loss.35 Bleeding risk is similar to tonsillectomy, which is approximately 4%. More significant issues such as velopalatine insufficiency, altered taste, tongue dysfunction and voice disturbance are uncommon.35
Future directions
Personalised therapy for OSA rather than a ‘one size fits all’ approach is important to address the pathophysiological causes of OSA at the individual patient level. This requires a phenotypic approach to diagnosis and management.
Combination therapy, in which two or more of the above non-CPAP treatment modalities are combined, is gaining momentum as an approach to enhance patient compliance and outcomes.
Hypoglossal nerve stimulation (HGNS) has been described as a surgically implantable, medically titratable device for treatment of OSA.48,49 Commercially available HGNS has been accessible for years elsewhere in the world,50 but remains at the clinical trials stage in Australia.
The search for a pharmacological solution for OSA is gathering momentum. Unravelling of the neuropharmacology of upper airway muscle control has identified a number of drug targets, including potassium channels, gamma-aminobutyric acid, and noradrenergic and serotonergic pathways.51 It has been suggested that therapy aimed at combined target receptors may improve OSA parameters.52
Conclusion
Patients with OSA benefit from a personalised and often multidisciplinary approach. CPAP is considered first-line treatment in the majority of patients; however, patients unable to tolerate CPAP or MAS therapy with favourable anatomy should be surgically assessed. New therapeutic modalities are being researched to define appropriate patient groups suitable for such treatment.
Key points
- GPs have a key role in diagnosis and management of OSA at a primary care level through directed history and clinical assessment. PSG and sleep physician referral may follow advice specific to weight loss, alcohol reduction, avoidance of supine positioning and healthy lifestyle.
- Trained, credentialed sleep physicians should guide CPAP and other therapies.
- Trained, credentialed sleep surgeons should guide surgical opinions, and similarly accredited dentists should guide MAS therapy options.