The recent Australian Exercise medicine in cancer management position statement recommendations for physical activity in cancer survivors,1 also supported by the Clinical Oncology Society of Australia position statement2 and the US guidelines,3 are in line with current recommendations for the healthy population: to perform at least 150 minutes of moderate-intensity aerobic exercise and 2–3 resistance training sessions per week. This equates to 30 minutes of moderate-intensity aerobic exercise, five days per week. Those unable to meet these physical activity recommendations should aim to be as physically active as possible. Importantly, the guidelines by Hayes et al take into consideration that it may not be possible for all individuals at different stages of their cancer journey to meet these guidelines. This is particularly relevant for individuals with lung cancer.
The terms ‘physical activity’ and ‘exercise’ are often used interchangeably, and it is relevant to define them here for clarity of use through this article. Physical activity is defined as ‘any bodily movement produced by skeletal muscles that results in energy expenditure’.4 Exercise is a subcategory of physical activity and is defined as physical activity that is ‘planned, structured, and repetitive, and has as a final or an intermediate objective the improvement or maintenance of physical fitness’.4
An increasing volume of research provides high levels of evidence to support the benefits of exercise for people with cancer to improve health-related quality of life (HRQoL), cancer-related fatigue and physical and social functioning.5–8 Studies report associations between higher levels of physical activity, improved survival and lower rates of cancer recurrence.9 The majority of this work has been conducted in patients with breast or prostate cancer and involves supervised, combined aerobic and resistance exercise. An individual patient data meta-analysis reports that supervised exercise programs result in the greatest health benefits when compared with unsupervised programs.5
The aim of the current review is to summarise what is currently known regarding the effects of the disease process, symptoms and treatments used to manage non–small cell lung cancer (referred to throughout as ‘lung cancer’) on physical activity and physical fitness. Physical activity and exercise intervention effects for people with lung cancer are presented at each stage of the treatment continuum. Patient exercise preferences and the use of behaviour change techniques in patient management are discussed. Recommendations regarding ways general practitioners (GPs) can support people with cancer to increase their physical activity are provided.
Why is exercise capacity reduced in lung cancer?
The majority of patients with lung cancer are diagnosed with inoperable disease. In Australia in 2011, 42% of patients were diagnosed with metastatic (stage IV) disease; for an additional 29% of patients with lung cancer, the stage was unknown.10 Physical fitness, or exercise capacity, is measured by the ability of the body to transport environmental oxygen to skeletal muscle mitochondria and is dependent on the combined functioning of the respiratory and cardiovascular systems, vessels, blood and skeletal muscle.11 There is a normal decline in exercise capacity associated with ageing, and the majority of people with lung cancers are diagnosed after the age of 65 years. In addition, people with lung cancer have reduced exercise capacity at diagnosis, compared with age-matched healthy populations.12 For people with lung cancer, a number of inter-related factors may contribute to impaired exercise capacity (Figure 1). The effects on the respiratory system of the tumour, certain chemotherapy agents, radiotherapy and surgery include reduced diffusing capacity, damage to lung parenchyma and vascular structures, and alveolar hypoventilation.13 Promisingly, much progress is being made in the area of immuno-oncology – the use of the body’s own immune anti-tumour response – as a treatment for advanced lung cancer. For patients with stage III and IV lung cancer, the National Comprehensive Cancer Network recommends immunotherapies as consolidation therapy. When compared with placebo following concurrent chemoradiotherapy, immunotherapies show significant improvements in progression-free and overall survival.14 Immunotherapies can be associated with immune-related adverse events, including pneumonitis.14
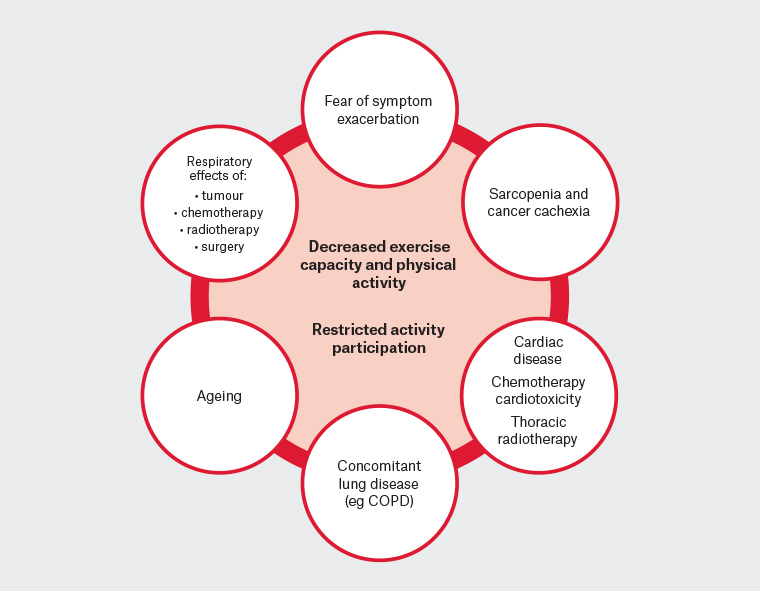
Figure 1. Factors contributing to reduced exercise capacity, physical activity and activity participation
COPD, chronic obstructive pulmonary disease
People with lung cancer frequently have concomitant smoking or asbestos exposure–related lung disease (eg chronic obstructive pulmonary disease [COPD], idiopathic pulmonary fibrosis) and cardiac disease.15 Impaired cardiac function is also a potential consequence of thoracic radiation, cardiotoxic chemotherapy agents and reduced haemoglobin levels.11 Skeletal muscle atrophy is common in individuals with lung cancer. Sarcopenia is highly prevalent (47%) in patients with stage III and IV disease treated with chemotherapy and is shown to be independently associated with an up to two-fold reduction in survival.16 Cachexia, defined as ‘a multi-factorial syndrome characterised by an ongoing loss of skeletal mass, with or without loss of fat mass, that cannot be fully reversed by conventional nutritional support and leads to progressive functional impairment’,17 occurs in up to one-third of patients with stage III and IV lung cancer.18 The combination of many of the above impairments leads to a multimorbid patient profile that may limit activities of daily living (ADLs) and participation in physical activity.
Symptoms of lung cancer and their impact on physical activity
People with lung cancer have different symptoms and symptom clusters as a result of the disease process, the treatments used to manage their cancer and associated comorbidities. These symptoms can significantly affect their capacity to perform ADLs as well as physical activity.19,20 One-third of people with lung cancer attribute major impairments in their ADLs to their diagnosis, and patient-reported symptoms have changed little over a 10-year period.21 The most commonly reported symptoms in those with newly diagnosed lung cancer include pain, fatigue, loss of appetite, coughing and sleep disturbance. Those with advanced lung cancer report high numbers of debilitating symptoms, most notably: pain, dyspnoea and anorexia. Higher levels of disease burden and distress associated with poorly managed symptoms are reported by more people with lung cancer than by any other tumour stream. Symptom severity is a prognostic indicator in lung cancer, with increased distress,22 fatigue and dyspnoea23 predictive of poorer prognosis.
Symptom management has important implications for improving other outcomes in this population, including physical function and HRQoL, where uncontrolled symptoms may contribute to people reducing their physical activity levels or avoiding certain activities for fear of exacerbating symptoms.20 Routine symptom self-reporting, with severe symptom ratings or significant increases triggering email alerts to hospital nurses, was found to result in reduced emergency department and hospital admissions, and improvements in chemotherapy tolerance, HRQoL and survival for patients with several types of metastatic cancer.24 Similar approaches to post-discharge symptom monitoring have been embedded into electronic health records in the UK for patients with cancer following surgery.25 Unfortunately, this approach to management has not been implemented into routine care in Australia. There is strong evidence that exercise reduces cancer-related fatigue.8 Similarly, pulmonary rehabilitation is effective in improving dyspnoea,26 and research continues into the effectiveness of non-pharmacological approaches to the management of dyspnoea including controlled breathing, cough easing and education.27 Exercise programs that include strengthening exercises can also address sarcopenia. Current research into managing cachexia shows a need for a multimodal approach that may include anti-inflammatory medication and nutrition advice (protein supplements) as well as exercise.28 We await outcomes of current international trials to better understand this aspect of management.
Physical activity and exercise interventions for patients with lung cancer
For people with lung cancer, exercise can be delivered safely prior to treatment (prehabilitation), perioperatively or postoperatively, during and following neoadjuvant or adjuvant treatment and in the palliative care setting (Figure 2). The following sections outline the evidence for exercise at different points on the cancer journey and provide information for GPs to support patients to engage in physical activity. This is still an emerging area of research and clinical practice, but the message from the evidence to date is consistently positive.
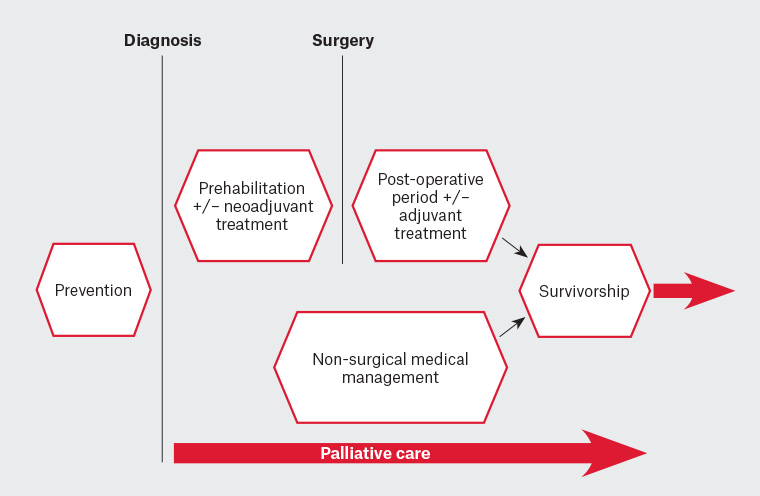
Figure 2. Options for the timing of exercise delivery across the lung cancer continuum of care
Lung cancer exercise prescription
Exercise testing and prescription for patients with lung cancer adheres to general principles, along with cancer-specific safety considerations that are provided in the recently updated American College of Sports Medicine (ACSM) guidelines29 and summarised in Table 1. To date, moderate-intensity aerobic exercise is predominantly prescribed without adverse events; one common example is walking, commenced at between 10 and 30 minutes per day. Resistance training can include exercise with the use of gravity, weights or functional exercises such as chair stands, step ups and squats. Care in prescription is needed in the presence of metastases and is best done by exercise professionals trained in exercise prescription for patients with cancer.
| Table 1. Cancer-specific considerations for healthcare professionals undertaking exercise testing and training for people with lung cancer29 |
| Issue |
Advice |
| Comorbid disease |
Complete screening in line with general guidelines and choose exercises that avoid exacerbation of symptoms, for example hydrotherapy. |
| Bony metastases |
Avoid strength assessment of muscles that attach to or act on a bony lesion site. Sites without metastases can be tested.
Advise patients to avoid weights involving the affected bones and try swimming. |
| Cardiovascular disease (premorbid or due to cancer treatment) |
Complete screening and perform cardiopulmonary exercise testing dependent on screening findings, as patients should obtain medical clearance prior to commencing exercise. |
| Peripheral neuropathy |
Assess balance, mobility and falls risk and modify exercise prescription as required (eg use a bicycle or water rather than a treadmill for aerobic exercise). |
| Lymphoedema |
Use of compression garments during exercise should be based on patient preference following education from exercise professionals. |
| Symptom clusters |
Refer to medical team when symptoms vary unexpectedly or result in safety concerns. |
| Infection and immunity |
Advise patients that exercising with a fever is contraindicated.
Precautions exist for patients with impaired haematological parameters; for example, patients with low neutrophil counts (≤0.5 × 10.9 cells/µL) should avoid public gyms. |
| Cancer cachexia |
Commence with prescription for resistance training only to increase muscle mass. |
| Note: The American College of Sports Medicine recommends that physician clearance and referral to an exercise professional is required in the following instances: bony metastases, lung/abdominal surgery, ostomy, cardiopulmonary disease, ataxia, extreme nutritional issues or fatigue, changing physical status (eg unstable lymphoedema). |
Exercise preferences of patients with lung cancer
Past exercise behaviour has a strong role in enabling future exercise (Table 2).30,31 The majority of patients with advanced lung cancer report motivation to exercise, with significant factors that increase exercise motivation including past exercise history, non-smoking status and absence of COPD.31 Reluctance to exercise has been reported when rehabilitation services were not seen as a priority or were viewed as being too burdensome or having little benefit, or when participants were waiting for other outcomes such as test results or treatment completion prior to considering rehabilitation.32 Generally, patients with newly diagnosed33 advanced cancer34 report preferences for home-based interventions consisting of walking. Positive experiences are reported by patients with lung cancer involved in home-based exercise after surgical resection35 and during treatment for inoperable disease.19 To support patient preferences to exercise effectively at home, greater use of technologies is needed so that exercise can be supervised and progressed for individuals.
| Table 2. Enablers and barriers to exercise for patients with lung cancer30 |
| Enablers |
Barriers |
- Expected benefits to physical and
mental health
- Improved symptoms
- Maintained independence
- Social support
|
- Perceived lack of benefit
- Fear of exercise
- Poor adaptation to diagnosis
- Poor prognosis
- Symptoms
- Presence of comorbidities
- Past sedentary lifestyle
- Poor access to exercise programs
|
Current evidence regarding physical activity and exercise interventions in lung cancer
Prehabilitation
International general cancer prehabilitation guidelines have recently been published outlining principles and steps to integrate prehabilitation into the standard cancer care pathway.36 A Cochrane review of five randomised controlled trials (RCTs) concluded that prehabilitation prior to surgical resection resulted in a reduction in the number of postoperative pulmonary complications (PPCs, 67% reduction), duration of intercostal catheter need and hospital length of stay.37 Following this review, a further six RCTs were published,38 with overall consistent findings to the prior review. The largest new trial of prehabilitation involved high-intensity interval training and reported no serious adverse events, with significant short-term improvements in pre-operative exercise capacity and reductions in PPCs favouring the exercise group.39 Feasibility of exercise training prior to commencement of radiotherapy has also been reported.40
Perioperatively and postoperatively
Systematic reviews of exercise interventions in people undergoing surgical resection +/– chemotherapy for lung cancer report safety, and data show short-term improvements in exercise capacity, quadriceps muscle strength, dyspnoea, HRQoL (physical component) and cancer-related fatigue, with few adverse events.41 Early postoperative supervised exercise is reported to be safe and feasible and resulted in significant improvement in exercise capacity and muscle strength in adherent participants.42 Exercise commenced earlier rather than later postoperatively is associated with greater reductions in cancer-related fatigue.43 A home-based physical activity intervention reported safety and feasibility during the postoperative period, with pre-operative physical activity levels maintained at eight weeks postoperatively.44
During and following non-surgical treatment
Few adequately powered RCTs are published to date examining exercise for patients undergoing non-surgical treatments, although several trials are nearing completion internationally.45,46 For patients with advanced lung cancer, a Cochrane review of exercise training, which included six RCTs, reported significant improvements in exercise capacity and disease-specific HRQoL favouring the intervention group but no differences in dyspnoea, mood or respiratory function.47 Included studies were rated as low quality. A home-based program of exercise, behaviour change and symptom management support did not change physical function but resulted in significant improvements in HRQoL (overall scores and physical, functional and symptom subscales) and symptom severity six months post-commencement of medical treatment in 92 patients with inoperable lung cancer.18 Further details regarding RCTs in the non-surgical population are provided in Table 3 (available online only).
| Table 3. Summary of exercise randomised controlled trials in advanced, inoperable lung cancer |
| Author, location and year |
Sample
size |
Cancer stage, type and medical treatment (Rx) |
Intervention |
Outcome measures and timing |
Results |
Hwang, Taiwan
20121 |
24 |
Stage: IIIA–IV NSCLC (83% stage IV)
Rx: targeted therapy for at least 1 month prior to recruitment. |
Setting: hospital outpatients Exercise type: supervised high-intensity aerobic (bike or treadmill)
Frequency: three times/week × eight weeks
Session duration: 30–40 minutes |
Outcomes: physical fitness, muscle strength and endurance, dyspnoea and HRQoL
Timing: pre- and post-program |
Completion: 18/24 (75%)
Adherence: 71%
Serious adverse events: nil
Findings: physical fitness increased in IG (P <0.005).Between-group changes in muscle strength, dyspnoea, HRQoL and fatigue were non-significant. |
| Henke, Germany 20142 |
46 |
Stage: IIIA–IV NSCLC or SCLC Rx: during three cycles of palliative chemotherapy |
Setting: hospital inpatients
Exercise type: continuous aerobic (walking and stairs), breathing techniques and RT
Frequency: aerobic × five days/week, strength every other day during admission |
Primary outcome: Barthel index (BI)
Secondary outcomes: HRQoL, physical fitness, stair walking, muscle strength and dyspnoea
Timing: pre- and post-program |
Completion: 29/46 (63%)Adherence/serious adverse events: NR
Findings: increase in BI (P = 0.003), single scores of HRQoL (physical functioning P = 0.025, haemoptysis P = 0.019, and arm/shoulder pain P = 0.048), physical fitness, stair walking, strength and dyspnoea perception (all P <0.05) favouring IG. |
Jastrzębski, Poland
20153
|
20 |
Stage: III–IV NSCLC or SCLC (or an earlier stage not suitable for radiotherapy or surgery)
Rx: during chemotherapy |
Setting: hospital inpatients Exercise type: continuous aerobic (Nordic walking or cycling), breathing techniques and RT
Frequency: aerobic × five days/week, respiratory and RT and breathing – daily, for 8–12 weeks
Session duration: aerobic and breathing 30 minutes and RT 30 minutes |
Outcomes: physical fitness, lung function, dyspnoea, HRQoL
Timing: pre- and post-program |
Completion: 100%
Adherence/serious adverse events: NR
Findings: IG showed a trend towards improved physical fitness (P = 0.252) and significant improvement in FEV1 (P = 0.016) and dyspnoea (P = 0.047). Marked reduction in HRQoL in both groups. |
Dhillon, Australia
20174 |
112 |
Stage: III–IV NSCLC or SCLC Rx: stage III or SCLC-LS; at least 4 weeks post-curative intent treatment with an incomplete response. Stage IV or SCLC-ED; palliative chemotherapy, chemotherapy plus targeted therapy, or targeted therapy |
Setting: outpatient hospital and home
Exercise type: physical activity and behaviour change; aerobic with advice about RT
Frequency: twice/week hospital, once/week home for eight weeks Session duration: hospital – 45 mins physical activity and 15 mins behaviour support |
Primary outcome: fatigue Secondary outcomes: HRQoL, functional abilities and physical function, anxiety and depression, sleep, distress, dyspnoea, physical activity and sedentary behaviour, hospitalisations, survival, cytokine and insulin-like growth factor levels and lung function
Timing: pre- and two, four and six months post-program |
Completion: 62/112 (55%) completed six-month outcomes
Adherence: 69% (physical activity) and 75% (behavioural) hospital sessions
Serious adverse events: nil
Findings: 95% (n = 106) NSCLC stage IV. No significant difference between groups at any timepoint. Exercise group increased self-reported physical activity, compared to control group (four months P = 0.039, six months P = 0.029) but this increase was not observed with objective measurement. |
Vanderbyl, Canada
20175 |
36 |
Stage: III–IV lung or gastrointestinal
Rx: receiving or scheduled to receive chemotherapy |
Setting: hospital outpatients
Exercise type: medical QG – group walking in a state of deep relaxation with coordinated arm movements and breathing pattern, SET – individual or group supervised continuous aerobic and RT, with education to walk at home
Frequency: twice/week for six weeks each group (cross-over trial)
Session duration: QG – 45 minutes, SET – NR; home exercise 60 minutes daily both groups |
Primary outcomes: mood and HRQoL
Secondary outcomes: functional assessment (including ADLs, sit to stand, 50-foot speed walk, physical fitness), symptoms and patient satisfaction
Timing: pre-program, end of first six weeks and completion |
Completion: 24/36 (67%) at six weeks and 19/36 (53%) at completion. Adherence: hospital – QG 75%, SET 87%, home – NR
Serious adverse events: NR
Findings: after first and second interventions neither group had significant changes in anxiety, depression or HRQoL. For the first intervention, compared with QG, SET group significantly increased physical fitness (P = 0.002), sleep quality (P = 0.03), wellbeing (P = 0.01) and feelings of strength (P = 0.01). Order of interventions had an effect – benefits of SET were not demonstrated in those completing as the second intervention. |
Edbrooke,
Australia
20196 |
92 |
Stage: inoperable NSCLC
Rx: during and following active treatment (chemotherapy and/or radiotherapy, targeted therapy, immunotherapy, stereotactic radiotherapy |
Setting: home-based
Exercise type: continuous aerobic (walking or cycling), RT (functional exercises including sit-to-stand, step-ups, squats, heel raises), behaviour change techniques to increase activity
Frequency: aerobic commenced at a minimum of 10 minutes twice weekly and progressed towards 150 minutes of moderate-intensity exercise per week; RT 2–3 sessions per week
Session duration: eight weeks of weekly physiotherapy home-visits (up to three) or telehealth session plus weekly symptom-management support nursing sessions using telehealth |
Primary outcome: physical fitness
Secondary outcomes: physical activity, quadriceps muscle and handgrip strength, HRQoL, mood, symptom severity and distress, exercise motivation, exercise self-efficacy and survival
Timing: baseline, nine weeks and six months |
Completion: 72% (66/92) at six months
Adherence: 65% aerobic exercise, 53% RT
Serious adverse events: nil
Findings: no significant between-group differences at 9 weeks. At 6 months significant between-group differences favouring the intervention group for HRQoL (overall, physical, functional and symptom subscales, P = 0.005), symptom severity (P = 0.001) and motivation to exercise (P = 0.041). Non-significant median survival benefit favouring the intervention group of 230 days (P = 0.15). |
Rutkowsa,
Poland
20197 |
40 (including participants from Jastrzębski et al) |
Stage: IIB–IV NSCLC
Rx: during chemotherapy |
Setting: hospital inpatients in two cycles between chemotherapy
Exercise type: aerobic (Nordic and treadmill walking or cycling) and breathing
Frequency: aerobic × five days/week, respiratory and RT and breathing – two × two-week training periods
Session duration: aerobic and breathing 30 minutes and RT 30 minutes |
Timing: baseline and six weeks |
Completion: 75% (30/40) at six weeks
Adherence: NR
Serious adverse events: nil
Findings: significant improvements favouring the IG in physical fitness, physical function and spirometry (all P <0.05). |
ADL, activities of daily living; ES, extensive stage; HRQoL, health-related quality of life; IG, intervention group; LS, limited stage; NSCLC, non–small cell lung cancer; NR, not reported;
QG, qigong; RT, resistance training; Rx, medical treatment; SET, standard exercise therapy; SCLC, small cell lung cancer
Serious adverse events reported are those related to trial exercise |
| References
1. Hwang CL, Yu CJ, Shih JY, Yang PC, Wu YT. Effects of exercise training on exercise capacity in patients with non-small cell lung cancer receiving targeted therapy. Support Care Cancer 2012;20(12):3169–77. doi: 10.1007/s00520-012-1452-5.
2. Henke CC, Cabri J, Fricke L, et al. Strength and endurance training in the treatment of lung cancer patients in stages IIIA/IIIB/IV. Support Care Cancer 2014;22(1):95–101. doi: 10.1007/s00520-013-1925-1.
3. Jastrzębski D, Maksymiak M, Kostorz S, et al. Pulmonary rehabilitation in advanced lung cancer patients during chemotherapy. Adv Exp Med Biol 2015;861:57–64. doi: 10.1007/5584_2015_134.
4. Dhillon HM, Bell ML, van der Ploeg HP, et al. Impact of physical activity on fatigue and quality of life in people with advanced lung cancer: A randomized controlled trial. Ann Oncol 2017;28(8):1889–97. doi: 10.1093/annonc/mdx205.
5. Vanderbyl BL, Mayer MJ, Nash C, et al. A comparison of the effects of medical Qigong and standard exercise therapy on symptoms and quality of life in patients with advanced cancer. Support Care Cancer 2017;25(6):1749–58. doi: 10.1007/s00520-017-3579-x.
6. Edbrooke L, Aranda S, Granger CL, et al. Multidisciplinary home-based rehabilitation in inoperable lung cancer: A randomised controlled trial. Thorax 2019;78(8):787–96. doi: 10.1136/thoraxjnl-2018-212996.
7. Rutkowska A, Jastrzebski D, Rutkowski S, et al. Exercise training in patients with non–small cell lung cancer during in-hospital chemotherapy treatment: A randomised controlled trial. J Cardiopulm Rehabil Prev 2019;39(2):127–33. doi: 10.1097/HCR.0000000000000410.
|
Palliative care
Patients receiving palliative care express a desire to exercise; however, patients cite an inability to attend hospital programs and a lack of mobility or energy as reasons for declining to participate.48 Qualitative research in patients completing a hospice-based exercise program revealed improvements in strength, balance and physical function; positive effects of group interaction and support from exercise professionals; and a sense of regaining control.49 Setting individualised treatment goals for these patients is essential, and the palliative care phase of management should not be a barrier to providing physical activity and exercise advice.
Physical activity interventions and behaviour change
Health behaviour change interventions, such as those targeting diet and physical activity, recognise the biological, psychological and social factors that affect an individual’s ability to change and maintain health behaviours. The motivation provided by a lung cancer diagnosis and the importance of health professional support to change behaviours are factors highlighted in recent research.50 The most commonly used behaviour change techniques in interventions to improve physical activity levels are collaborative health-related goal setting, instruction regarding how to perform the behaviour and action planning.51 Use and reporting of behaviour change techniques is suboptimal in thoracic cancer exercise studies, making it difficult to assess the efficacy of these techniques in improving physical activity.51
The role of general practitioners
It is recognised that doctors discussing physical activity with their patients regularly is key to increasing the importance patients place on exercise as part of their cancer management. The ‘Exercise is Medicine’ ACSM initiative aims to support clinicians to make exercise referrals and routinely evaluate physical activity levels.52 The initiative outlines the role of the clinician in three stages:
- Assess – ask patients about their current physical activity as a routine part of assessment of vital signs
- Advise – encourage patients to increase physical activity if they are not meeting recommendations and are considered safe to exercise without medical supervision (a downloadable exercise template can be found at www.exerciseismedicine.org/movingthroughcancer)
- Refer – refer patients to an exercise professional if the patients are not safe exercising without supervision or you are unsure.
Physical activity and exercise interventions provided in centres or at home are safe prior to, during and following all treatments for lung cancer when prescribed and monitored by an exercise professional. Individuals with lung cancer should be encouraged to be as physically active as possible and avoid sedentary time. Prescription should be individualised according to patient preferences and abilities, as well as symptom interference and medical safety. Support to manage uncontrolled symptoms and use of behaviour change strategies are key to reducing barriers to physical activity participation for all people with a cancer diagnosis, including those with lung cancer. Addressing nutritional issues proactively as part of cachexia prevention/treatment is important (Box 1).
| Box 1. The general practitioner’s role in supporting patients with lung cancer to be physically active |
- Encourage increased physical activity and reduced sedentary time across the whole cancer journey
- Support setting of patient-individualised activity goals
- Involve family and caregivers in activity and nutrition advice and goal setting
- Assess symptoms and assist with advice to reduce their impact
- Understand safety criteria for exercise
- Refer to an exercise professional and/or dietitian for exercise and/or nutrition advice
- Provide access/references to education materials/online websites
- Empower patients to take control of their own care
|
Conclusion
Research supports the positive effects of increased functional exercise capacity on cancer-related fatigue, physical function, symptom distress, sarcopenia and HRQoL. However, an immediate challenge is access to education and educational materials to assist GPs. Additionally, referral to exercise professionals is not always easy, and a database of those with an interest in oncology would be of assistance. Finally, there are few exercise programs specifically for patients with cancer in the acute sector or community, although these are now increasing with emerging supportive evidence for efficacy. Patients with lung cancer can be referred to pulmonary rehabilitation programs. Advocacy to increase access to rehabilitation exercise treatments for patients with lung cancer as well as to improve GP educational resources through The Royal Australian College of General Practitioners is encouraged to improve the primary care management of cancer generally and lung cancer in particular.
Resources
Exercise program examples and finding healthcare professionals in your area