Hypertension is the most common problem encountered in general practice.1 Primary aldosteronism is the most common specifically treatable cause of hypertension. It affects 5–10% of patients with hypertension in primary care and up to 30% of those with refractory hypertension.2,3 Despite this, screening for primary aldosteronism is infrequently performed in primary care.3 This may be partly due to lack of guidance regarding how to adjust medications prior to screening.
The most common causes of primary aldosteronism are bilateral adrenal hyperplasia (BAH) and aldosterone-producing adrenal adenoma (APA).2 Unilateral APAs are curable by laparoscopic adrenalectomy, whereas BAH can be managed using targeted medical therapy in the form of mineralocorticoid receptor antagonists such as spironolactone or eplerenone, which block the effects of aldosterone.2 Specific treatment of patients with primary aldosteronism by adrenalectomy or targeted medical therapy as appropriate has been associated with a reversal of left ventricular hypertrophy, as well as a reduction in associated complications such as cardiovascular disease, atrial fibrillation and proteinuria.4
Aetiology of hypertension in primary aldosteronism
In a series of elegant experiments, Arthur Guyton showed that long-term arterial pressure is the function of the renal pressure natriuresis curve.5 Salt intake causes fluid retention, fluid retention raises arterial pressure, and this increases pressure-driven natriuresis to match the salt intake.5 Matching natriuresis to sodium intake allows the kidney to maintain an acceptable blood pressure over the long term despite a wide variation in dietary sodium.5
With increased aldosterone, the pressure–natriuresis curve is shifted rightwards and rotated clockwise.6 Stated another way, the arterial pressure will be higher for any given concentration of sodium intake when serum aldosterone is elevated. The result is that excessive aldosterone produces a hypertensive state characterised by increased plasma volume, total body volume and total peripheral resistance.
Aldosterone also induces pathological changes independent of hypertension in the heart, kidneys and other organs.7 This likely explains why patients with primary aldosteronism have a higher risk of stroke, atrial fibrillation, acute myocardial infarction and heart failure when compared with blood pressure–matched essential hypertension.7 Additionally, this observation likely explains why mineralocorticoid receptor antagonists improve end-organ damage parameters above and beyond what would be expected for the blood pressure reduction alone.
Brief overview of screening for secondary hypertension
Although primary aldosteronism is the focus of this article, it is worth providing an overview of when to screen for some other causes of secondary hypertension in adults. There are specific signs and symptoms that characterise some causes of secondary hypertension, which have been detailed in recent literature.8–10 Cushing’s syndrome is suggested by the clustering of comorbid insulin resistance, acne, central adiposity, capillary fragility, moon facies and/or the known use of high-dose exogenous corticosteroids.8–10 There are multiple aetiologies for this syndrome, including Cushing’s disease (an adrenocorticotropic hormone–producing pituitary adenoma).8–10 Pheochromocytoma is rare but should be considered in a patient with headache, tachycardia, sweating, pallor and hypertension.8–10
Renovascular hypertension does not tend to manifest any unique symptoms but should be considered when significant hypertension occurs prior to the age of 30 years, where: 8,10
- there is a significant decline in renal function following angiotensin-converting enzyme (ACE) inhibitor or angiotensin receptor blocker (ARB) use
- hypertension is present with significant renal size asymmetry
- there is an accelerated and severe progression of the hypertensive state.
Obstructive sleep apnoea is a common contributing factor to hypertension, but any patient with hypertension and obstructive sleep apnoea should be screened for primary aldosteronism.8,10
Who should be screened for primary aldosteronism?
The question of whom to screen has been reviewed in a recent article by Lim et al.11 Box 1 summarises the criteria that should prompt case-finding for primary aldosteronism.
| Box 1. The Endocrine Society’s current screening recommendations for primary aldosteronism2,11 |
- Sustained blood pressure (BP) above 150/100 mmHg on each of three measurements obtained on different days, or
- Hypertension (BP >140/90 mmHg) resistant to three conventional antihypertensive drugs (including a diuretic), or
- Controlled BP (<140/90 mmHg) on four or more antihypertensive medications
- Hypertension and spontaneous or diuretic-induced hypokalaemia
- Hypertension and adrenal incidentaloma
- Hypertension and sleep apnoea
- Hypertension and a family history of early-onset hypertension or cerebrovascular accident at a young age (<40 years)
- All hypertensive first-degree relatives of a patient with primary aldosteronism
|
| Reproduced with permission from Lim Y, Shen J, Fuller P, Yang J, Current pattern of primary aldosteronism diagnosis: Delayed and complicated, Aust J Gen Pract 2018;47(10):712–18. doi: 10.31128/AJGP-05-18-4587. |
Screening can lead to either curative surgery or targeted antihypertensive therapy, both of which will improve blood pressure control, reduce medication burden and decrease the rate of end-organ damage.7 Even for elderly patients or those who are not candidates for surgery, screening may facilitate the effective use of mineralocorticoid receptor antagonist treatment without resorting to more invasive investigations to fully characterise the disease.
What is the optimal screening protocol for primary aldosteronism?
An aldosterone to renin ratio (ARR) measured at least two hours after arising in the morning is the appropriate screening test.2 The test does not require fasting, though a fasting sample is still valid. An ARR >70, where plasma aldosterone concentration is measured by radioimmunoassay and expressed in pmol/L and direct renin concentration is measured by chemiluminescent assay and expressed in mU/L, is considered a positive screening test. Screening is best performed prior to starting any antihypertensive medications. This is because the most commonly used antihypertensive medications affect either aldosterone or renin concentration, and therefore undermine the accuracy of screening.2
For patients who have commenced antihypertensive medications, the gold standard for accurate screening would be to cease all interfering medications and replace them with medications that have minimal effect on aldosterone and renin concentration. This is not always practical, so stopping certain medications should be prioritised over others (Table 1), with guidelines varying as to the degree of importance placed on this.12 The approach outlined here recognises the importance of avoiding inaccurate testing but also acknowledges that sometimes it is too difficult to completely optimise medications.
| Table 1. How to prioritise medication changes prior to screening for primary aldosteronism |
| Group |
Medications |
Group 1: Must be replaced for accurate screening
|
All loop diuretics (eg frusemide, bumetadine)
All thiazide diuretics (eg hydrochlorothiazide, indapamide, chlortalidone)
All mineralocorticoid receptor antagonists (eg spironolactone, eplerenone) |
Group 2: Replace
wherever possible
|
All angiotensin-converting enzyme inhibitors (eg perindopril)
All angiotensin receptor blockers (eg olmesartan)
All dihydropyridine calcium channel blockers (eg amlodipine) |
| Group 3: Replace only after addressing medications in Groups 1 and 2 |
Selective and non-selective beta blockers (eg atenolol) |
Interfering medications should be ceased and replaced with sustained-release verapamil, prazosin, moxonidine and/or hydralazine in the order of priority suggested by Table 1.12,13 These new medications can be started the same day that the other antihypertensive medications are ceased, with caution advised when changing from a beta blocker to verapamil so as to include a period of weaning before switching medication. The new medication regimen should be continued for six weeks prior to screening. Table 2 provides practical points regarding using these less commonly prescribed antihypertensive medications.
| Table 2. Medications that do not affect screening and how to use them |
| Medication |
Dose |
Practice points |
| Sustained-release verapamil |
180 mg orally daily, up to 240 mg orally daily16 |
Must be sustained release.
Higher doses produce adverse effects. If not at target, add another agent.
Common adverse effects include constipation and bradycardia. Infrequently, it can cause or worsen atrioventricular (AV) blockade and heart failure.16 |
| Moxonidine |
200 μg once at night, can increase to 200 μg twice daily after two weeks16 |
Only eligible on the Pharmaceutical Benefits Scheme as a second antihypertensive.
Contraindicated in heart failure, bradycardia, AV block and creatinine clearance <30 mL/min.16
Common adverse effects include dry mouth, somnolence, dizziness and weakness.16 |
| Prazosin |
0.5 mg orally twice daily, increase up to 5 mg orally three times per day16 |
Up-titrate slowly to avoid postural hypotension.
Avoid in people with symptomatic cataract requiring surgery.
Common adverse effects include dizziness, palpitations, dry mouth and blurred vision. Infrequently, tachycardia, urinary incontinence and fainting may occur.16 |
| Hydralazine hydrochloride |
12.5 mg orally twice daily, can increase up to 50 mg orally three times per day16 |
Common adverse effects include flushing, headache, palpitations, oedema and dizziness, while angina and a lupus-like syndrome can occur infrequently.16 |
These authors’ experience suggests that if the patient has no significant end-organ damage, then the process of switching medications is usually straightforward. Patients who have existing nephropathy or ischaemic heart disease can continue to be screened in a primary care setting, but more frequent blood pressure and adverse effect monitoring will be required. For patients with heart failure and a current left ventricular ejection fraction of <50%, ACE inhibitors and ARBs should not be withdrawn.
For patients who are too elderly or frail, or have strong indications for their existing antihypertensive therapy, their ARR can be measured without switching medications.12 However, the results will need to be carefully interpreted. For example, if a patient has a low or low-to-normal blood renin concentration despite taking an ACE inhibitor or ARB (which generally increase renin), then one should be highly suspicious of underlying primary aldosteronism. Hypertension specialist advice may be sought in this setting, including whether it is better to forego additional investigations and instead opt for a trial of mineralocorticoid receptor antagonist therapy on the basis of an abnormal or suspicious ARR.
Serum potassium should be corrected if it is low.2 As previously mentioned, ACE inhibitors and ARBs raise serum potassium. If starting potassium supplements for hypokalaemia (eg 600 mg slow-release potassium chloride, one per day), it is important to recheck the potassium a week later to ensure it has normalised and, if it has not, increase the dose. The ARR is best performed six weeks after optimising the medications and normalising the potassium.
Other medications that can affect the ratio have not been mentioned in detail. These include antidepressants, the combined oral contraceptive pill (COCP) and hormone replacement therapy (HRT).2 Antidepressants tend to be continued because of the practical difficulties of stopping them. The Royal Australian and New Zealand College of Obstetricians and Gynaecologists guidelines state that the COCP is not recommended for use in the setting of hypertension; for example, in the setting of primary aldosteronism work-up, the risks of using the COCP would generally outweigh the benefits.14 On a related note, any changes to HRT treatment would be carefully considered on a case-by-case basis.
Following up a positive screening test
After a positive screening test, treatment priorities are:
- Ensuring the patient continues taking the new medications
- Ensuring the patient maintains a normal serum potassium. Slow-release potassium supplements are ideal to avoid transient hyperkalaemia.
- Explaining to the patient what comes next. The Endocrine Society has produced an extremely user-friendly guide to the patient journey from positive screening test to curative surgery (and everything in between).15 The full link can be found in the clinical guidelines section of the Endocrine Society website.2
Putting it all together: Practical pointers
Figure 1 provides an algorithm for screening a patient who is already taking antihypertensive medications. This reflects the ideal scenario. A composite case study has also been provided.
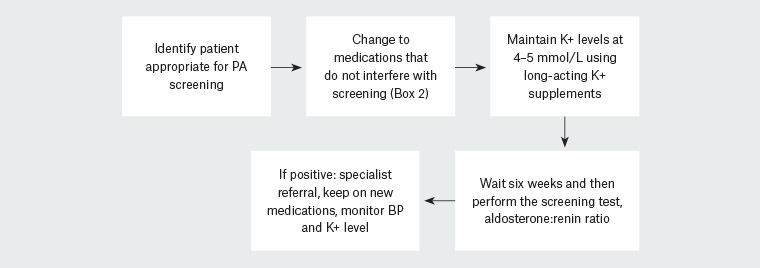
Figure 1. Summary of the primary aldosteronism screening process when the patient is taking interfering medications
BP, blood pressure; K+, potassium; PA, primary aldosteronism
Case: Screening for primary aldosteronism
JJ, a male smoker aged 48 years, presented requesting a prescription for his regular medications: perindopril 10 mg daily, amlodipine 10 mg daily, atorvastatin 40 mg daily and aspirin 100 mg daily.
He reported taking medication since the age of 30 years because of a consistent systolic blood pressure (SBP) >160 mmHg, and said that his SBP ranged from 130–175 mmHg at home.
A recent serum potassium concentration of 3.4 mmol/L was noted in the medical record. Even in the absence of the hypokalaemia, JJ would meet the Endocrine Society criteria for primary aldosteronism screening because of his consistent pre-treatment SBP >150 mmHg. The hypokalaemia in the setting of hypertension meets an additional criterion to support primary aldosteronism screening.
Given JJ’s elevated body mass index (33 kg/m2), a sleep study was arranged, but no likely cause for secondary hypertension was suggestive on clinical review.
Because perindopril and amlodipine affect the accuracy of the ARR, these needed to be replaced with medications that do not affect testing. On the same day that perindopril and amlodipine were ceased, JJ commenced sustained-release verapamil 180 mg once daily, hydralazine 12.5 mg twice daily and 1200 mg slow-release potassium chloride twice daily. Additionally, aspirin was ceased as it is not indicated for primary prevention of cardiovascular disease. A follow-up appointment was made, with a repeat potassium measurement to be taken beforehand.
Three days later, JJ’s SBP was 172 mmHg and serum potassium was 3.4 mmol/L. Hydralazine was increased to 25 mg twice daily. Slow-release potassium chloride was increased to 1800 mg twice a day. Three days later, the serum potassium was 4.0 mmol/L, which was at target. JJ’s SBP then fluctuated between 150–165 mmHg, and hydralazine was increased to 25 mg three times per day, which brought the SBP into an acceptable range of 130–150 mmHg.
Following six weeks of treatment with non-interfering medications and having a normal serum potassium concentration, a screening ARR was performed. The result was an ARR of >216, which is well above the cut-off of 70. JJ was referred to the local endocrine hypertension unit, where he underwent a saline suppression test to confirm the diagnosis of primary aldosteronism, followed by adrenal vein sampling, which showed lateralisation of his aldosterone production to the left adrenal gland. A transthoracic echocardiogram showed moderate left ventricular hypertrophy.
Subsequently, JJ underwent retroperitoneal laparascopic left adrenalectomy. Eighteen months later, the left ventricular wall size, serum potassium and ARR were all normal, and he was able to maintain a blood pressure of 120/70 mmHg without antihypertensive agents.
Conclusion
Primary aldosteronism is the most common cause of secondary hypertension. Detection of accurate primary aldosteronism is achievable in primary care by replacing medications that affect the ARR with medications that do not, and ensuring the serum potassium concentration is normal. The process of medication switching can be safely achieved for the majority of patients. If the screening test is positive, referral to a specialist hypertension unit is indicated. Screening can lead to either curative surgery or targeted antihypertensive therapy, both of which will significantly improve blood pressure control, reduce medication burden and decrease the rate of end-organ damage caused by hypertension and aldosterone excess.