Case
A man aged 60 years presented with an irregularly shaped erythematous scalp plaque with a 5 cm diameter (Figure 1). Dermoscopy revealed white scar-like features and coiled vessels (Figure 2). The remaining scalp was heavily sun damaged with numerous actinic keratoses. The lesion had been treated previously with liquid nitrogen cryotherapy and photodynamic therapy (PDT). A shave biopsy measuring 16 mm × 7 mm × 1 mm confirmed an intraepidermal carcinoma (IEC); the base was not transected and there was no invasion.
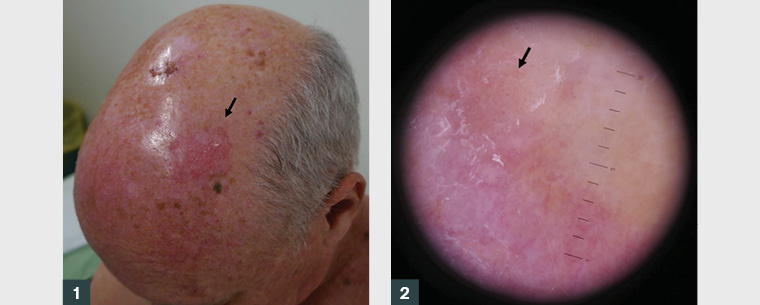
Figure 1. Large >5 cm intraepidermal carcinoma on the scalp (arrow).
Figure 2. Pink scaly plaque of intraepidermal carcinoma with dermatoscopic features of linearly arranged ‘coiled’ vessels (arrow)
Question 1
What is an IEC and how is it diagnosed?
Question 2
What are the available treatment options for IEC?
Question 3
What factors are associated with treatment resistance or recurrence?
Question 4
What would be your management approach for this lesion?
Question 5
What are the surgical management considerations in this case?
Answer 1
An IEC or squamous cell carcinoma in-situ (SCCIS) is a common form of superficial keratinocyte skin cancer with a predilection for sun-exposed areas in fair-skinned individuals. Cumulative ultraviolet radiation exposure is the main aetiological factor; other risk factors include human papillomavirus, exposure to radiation therapy, immunosuppression, arsenic and polycyclic aromatic hydrocarbons in industrial coal tar and petrochemicals.1 An IEC most commonly presents as a well-demarcated erythematous scaling macule, patch or thin plaque. Histopathology reveals full-thickness atypia of the epidermis but no invasion into the underlying dermis. Variants include hypertrophic, pigmented, nail unit and genital lesions. Some will show adnexal invasion.2 Progression to invasive squamous cell carcinoma (SCC) may occur in 5% of lesions if left untreated.3
Answer 2
There are multiple therapeutic options available for IEC, and some may be used in combination. No single treatment is superior for any one situation. Recurrence rates following topical and locally destructive modalities have been reported to vary from 3% to 35%.4 Table 1 lists average response rates in parentheses.
| Table 1. Average clinical clearance rates following treatment of intraepidermal carcinoma |
| Topical |
Locally destructive |
Surgical |
- 5-Fluorouracil (85%)3
- Imiquimod (73%)3
|
- Liquid nitrogen cryosurgery (61–86%)3,17
- Photodynamic therapy (85%)18
- CO2 ablative laser (inadequate data)
- Radiation therapy (93%)19
|
- Curettage and cautery (90%)3,18
- Excision (90–98%)3,20
- Excision with margin-controlled surgery (91–97%)3,21
|
Answer 3
Follicular or adnexal invasion and hypertrophic types may not be cleared with superficial treatment modalities.2 High-risk features of recurrence in invasive SCC such as lesion location (lips, subungual, anogenital), size >20 mm, tumour recurrence, localisation within a scar or prior irradiation and immunosuppression also present challenges when treating IEC.3,4 Referral to a specialist or multidisciplinary team for assessment should be considered for patients with these features.5
Answer 4
For a large scalp lesion that has recurred after previous PDT and cryotherapy, agents with a similar cure rate (eg 5-fluorouracil [5-FU] or imiquimod) are unlikely to perform any better. Curettage performs best for primary lesions <2 cm in diameter and would be a poor choice for a recurrent lesion.6 Radiation therapy is indicated for patients who are poor surgical candidates. In general, radiation therapy is not recommended for younger patients because of worsening functional and cosmetic results with time and the risk of developing a secondary cancer in the treatment field.7 The best option here is complete excision to ensure clear histological margins.
Answer 5
Some form of margin control could be used, including frozen section or Mohs surgery. Either of these would lengthen the operative time and cost for a relatively low-risk lesion. Scalp defects up to 3 cm in diameter can potentially be closed directly.8 Complete excision of this lesion will produce a wound that will need either a skin graft or complex flap repair.
A rotation flap extending to the posterior neck as dotted in Figure 3 was dismissed in favour of a split-thickness skin graft (STSG) to avoid distortion of uncertain margins. Re-excision is more straightforward where a graft is used, and a flap revision may be considered after clear histology if required for cosmesis or functional reasons.
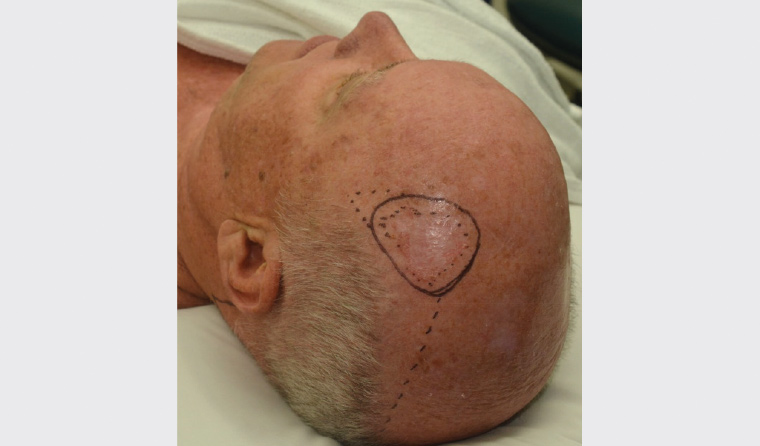
Figure 3. Pre-operative markings of large scalp intraepidermal carcinoma
STSG usually has a better ‘take’ rate and can cover a larger surface area than a full-thickness skin graft (FTSG) in a region such as the scalp because of reduced metabolic requirements. Both require a well-vascularised wound bed with intact periosteum.9 The donor site should be carefully selected to match the recipient site while considering potential donor site morbidity including pain, scarring, infection and dyspigmentation.
Factors considered when choosing the most appropriate closure option include: the size of the defect; its depth and anatomical plane; the pathology being treated and risk of recurrence; aesthetic considerations such as contour, soft-tissue thickness and effect on hair growth; the patient’s general medical condition and factors such as immunosuppression and prior radiotherapy.8
Case continued
The patient agreed to excision and closure with an STSG harvested from the thigh. A 4 mm clinical margin was used after examining the lesion with magnification as this was considered adequate to ensure complete excision. Histology confirmed a completely excised IEC with no evidence of invasion.
Question 6
What is your follow-up and preventive approach in this patient?
Answer 6
Educating the patient on sun protection and self-examination, enrolling the patient in long-term surveillance looking for recurrence and new cutaneous malignancies, and addressing the remaining ‘field’ of actinic damage damage are recommended following treatment.10 Most local recurrences for high-risk keratinocyte tumours occur within 2–3 years.11 Chemoprevention with low-dose systemic retinoids and nicotinamide (vitamin B3) are considered in organ transplant recipients and high-risk individuals with multiple keratinocyte cancers.12,13
Several options are available for the field treatment of actinic keratoses including 5-FU, imiquimod, ingenol mebutate, PDT and CO2 laser.14 While these have shown benefit in reducing actinic keratoses counts, their impact on preventing SCC is uncertain. A recent study combining 0.005% calcipotriol ointment with 5% 5-FU cream twice daily for four days demonstrated a significant reduction in the number of actinic keratoses on the face and scalp by eight weeks.15 Encouragingly, this demonstrated a reduction in invasive SCC at three years’ follow-up.16
Case continued
A four-week course of treatment with 5-FU to the patient’s scalp was prescribed six months following surgery, with a significant clearance of actinic keratoses in the treated field and no recurrence of IEC at two years (Figure 4).
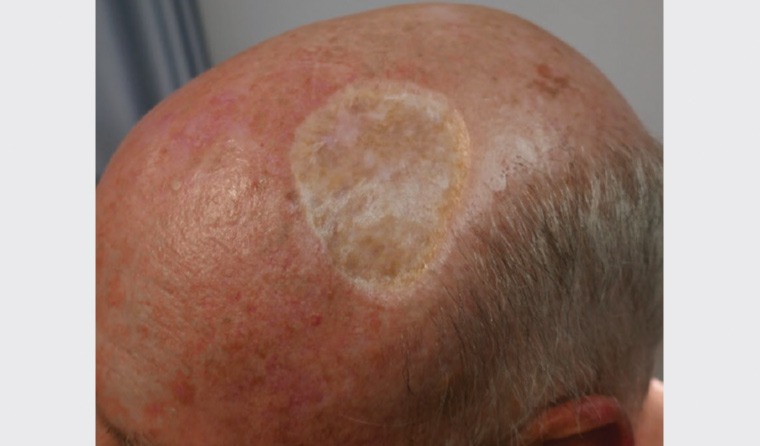
Figure 4. Two years after surgery; note the clearance of actinic keratoses following 5-fluorouracil field treatment
Key points
- When treating recurrent skin malignancy, it is important not to persist with a ‘losing’ modality and rather select one with a higher long-term cure rate.
- Larger IECs are more likely to recur.
- Complex closures should be avoided where margins are uncertain.
- Referral to a specialist or multidisciplinary team for assessment and treatment should be considered for patients with high-risk and recurrent tumours.
- National Health and Medical Research Council guidelines on the diagnosis and management of keratinocyte cancers are regularly revised and are available online from The Cancer Council Australia.