Nutrition plays a vital role in optimising bone mineral density (BMD) throughout life to reduce the risk of osteoporosis and osteoporotic fractures.1 Current Australian dietary recommendations for osteoporosis prevention in general practice are confined to promoting adequate intake of the individual nutrients calcium and protein.2 However, individual nutrients and food items are consumed together in the diet and potentially interact in complex ways.3 Dietary patterns can be used to investigate the effects of this on health outcomes3 as well as potentially provide evidence that is easier to translate into practice.4 One way of doing this is to identify dietary patterns from dietary intake data using approaches such as factor analysis.5
Individual studies investigating associations of empirically derived dietary patterns with bone density and fractures have produced conflicting results.6 A robust systematic synthesis of studies is needed that examines evidence separately for different clinically important fracture and bone density sites. The latter is important as fracture risk factors differ for different sites,7 as do the costs and sequelae of fractures.8 Failing to examine fracture or BMD/content outcomes by site is a significant limitation of one systematic review9 addressing this issue. This, and another review,10 also used risk of ‘low BMD’ as an outcome, where this was defined in different ways in different populations, making its clinical interpretation difficult, and in one case resulted in excluding information from relevant studies that did not use this outcome.10 Therefore, the aim of this systematic review was to determine whether empirically derived dietary patterns are associated with bone density and fracture outcomes at key clinically important sites, namely the hip, lumbar spine and forearm in healthy adults.
Methods
This review was prospectively registered on PROSPERO, the International Prospective Register of Systematic Reviews (Registration number CRD42017071676).
Literature search
The electronic bibliographical databases Medline and Embase were searched via OVID, CENTRAL (Cochrane) and Proquest: theses and dissertations from their inception to 12 May 2017 using key words relating to dietary patterns, BMD and fracture. The search was limited to adults, English language and human subjects. The full search strategy for each database is given in Appendix 1.
Selection criteria
The study included quantitative observational or intervention studies of any design that reported associations between dietary patterns and bone density outcomes and/or fractures. Studies were included if they were published in English, as full text and were peer-reviewed, and if participants were adults aged 18 years and over, who did not have diseases and were not taking medications affecting bone metabolism. The researchers included studies that derived dietary patterns using an empirical approach (eg factor analysis, principal component analysis, cluster analysis) on data from a validated dietary intake measurement.11 Dietary pattern scores could be calculated using any method. Studies were included if they measured bone density and/or fractures. For bone density, the researchers included studies that measured areal or volumetric BMD, or bone mineral content (BMC) using dual-energy X-ray absorptiometry (DEXA), single photon absorptiometry, dual photon absorptiometry, peripheral quantitative computed tomography, or broadband ultrasound attenuation and ultrasonic speed of sound by quantitative ultrasonography. Studies had to measure at least one of the following sites: femoral neck, total hip, total body, lumbar spine, proximal or distal forearm. Studies that reported total fracture and/or hip, distal forearm or radius and clinical (symptomatic) or radiological vertebral fracture fractures were included.
Study selection and data extraction
Two authors (HHN and FW) independently screened titles and abstracts of potential articles against the inclusion and exclusion criteria, and further assessed the full text if required. Disagreements were resolved by consensus. Two authors (HHN and JKM) independently extracted the following:
- study characteristics – title, author, study design, study date and duration, sample size and source population
- participant characteristics – age, sex, ethnicity, criteria for inclusion and exclusion
- methods of measuring dietary intake, determining dietary patterns and calculating diet pattern scores
- sites, methods and time points of measurement of BMD and/or fracture
- methods (including any adjustment for potential confounders) used to test for associations between outcomes and exposures and their results.
Assessment of methodological quality of included studies
HHN and JKM independently assessed the methodological quality of included studies using an approach developed for observational studies on musculoskeletal topics12 that can be modified for a specific topic, as the researchers had previously done.13,14 This approach has criteria assessing both the internal validity and informativeness of each study as given in Part A of Appendix 2. The number of criteria used for each study depended on the outcome measures: 19, 20 and 22 criteria were used if the study measured BMD, fracture or both respectively. For each study, each criterion was assessed as adequate if met (+), inadequate if not met (−), unclear (?) or not applicable (NA). The full list of criteria is included in Part B of Appendix 2. A quality assessment score was calculated by summing the number of criteria met (+), divided by the applicable number of criteria, multiplied by 100 to generate a percentage. Studies with a methodological assessment score ≥60% were considered to be high quality.12
Data synthesis
For each included study, tables were used to summarise key study and participant characteristics, methods of assessing dietary patterns and bone outcomes, and key methods and results of tests of associations between dietary patterns and bone outcomes. Because substantial clinical and methodological heterogeneity were expected in the included studies, the first step was to consider from these tables whether the studies reporting data for each outcome were acceptably similar for meta-analysis to be possible or best-evidence synthesis to be appropriate. A major consideration was judging the extent to which different dietary patterns were sufficiently similar to each other. This was undertaken by consensus between four authors (HHN, TW, FW, WHO), one of whom (WHO) is a Professor of Nutritional Epidemiology, by examining the composition of all the published dietary patterns in each study. The two categories of dietary patterns with the most consistently observed similarities were termed ‘healthy’ and ‘Western’ patterns as the closest reflection of their content, though they were named differently across studies (as summarised in Appendix 3). The ‘healthy’ group of patterns was characterised by high consumption of fruits, vegetables, wholegrains, nuts, legumes and fish, and the ‘Western’ group of patterns by high consumption of meats, processed meats, sweets including cakes or desserts, fats/oils, soft drinks and takeaway foods. There were enough data available for meta-analysis for only one outcome and exposure (hip fracture and ‘healthy’ pattern).
A best-evidence synthesis for both ‘healthy’ and ‘Western’ dietary patterns was therefore instead performed for each bone density site (femoral neck, total hip, lumbar spine, total body and forearm BMD and total body bone mineral content [TBBMC]) and total fracture and for ‘Western’ pattern and hip fracture. The levels of evidence were classified into five categories according to the criteria of Lievense et al.12 Evidence was considered strong if there were consistent findings in multiple high-quality cohort studies; moderate if the general consistent findings were shown in a single high-quality cohort study and two or more high-quality case-control studies, or three or more high-quality case-control studies; limited if consistent findings were shown in a single cohort study, one or two case-control studies, or multiple cross-sectional studies; conflicting if fewer than 75% of studies had consistent findings and as no evidence if no studies were found.
The meta-analysis of ‘healthy’ dietary pattern and hip fracture used the number of events and total number of participants in the highest versus lowest categories of healthy pattern scores (variously tertiles, quartiles and quintiles) to estimate pooled risk ratio (RR) and 95% confidence interval (CI), using random effects modelling. The researchers performed a subgroup analysis by method for measuring fracture (confirmed by medical record versus self-report). Heterogeneity was assessed using I-square (I2). Review Manager (RevMan) software version 5.3 (The Nordic Cochrane Centre, Copenhagen) was used for meta-analysis.
Results
Characteristics of included studies
From 1750 potential articles, 23 studies were included in the systematic review (Figure 1). Two were excluded from the best-evidence synthesis as they did not identify dietary patterns comparable to ‘healthy’ or ‘Western’ patterns. The characteristics of all included studies are given in Table 1. There were 12 cross-sectional studies (52.2%),3,15–25 10 cohort studies (43.5%)26–35 and one case-control study (4.3%).36 Sample sizes ranged from 1543 to 112,845.32 All studies were considered high quality. Diet was mostly measured using food frequency questionnaires (FFQ)3,15,16,18,19,24–36 and dietary patterns were generally identified by principal component analysis.3,17–25,29,30,32,34–36 Dietary pattern scores were mostly calculated using the weighted sum score method.3,16,19–22,24,27,29,34–36 BMD was most commonly measured by DEXA,3,16–26,31,33,34 and lumbar spine,3,17–24,31 femoral neck3,15,18,19,21,22,26,34 and hip18,20,24,31 were the most commonly measured sites. Fractures were mainly assessed from medical records27,30,33,35,36 and hip fracture30,32,33,35,36 was the most common site measured.
| Table 1. Characteristics of included studies (n = 23) |
| Characteristic |
n (%)* |
| Sample size: median (IQR) |
1818 (527–5188) |
| Study design |
|
| Cross-sectional |
12 (52) |
| Cohort |
10 (43) |
| Case–control |
1 (4) |
| Sex |
|
| Women only |
10 (43) |
| Mixed sexes |
13 (57) |
| Age groups |
|
| Elderly (≥50 years) |
12 (52) |
| Mixed (younger and elderly) |
10 (43) |
| Younger (<50 years) |
1 (4) |
| Method of dietary intake |
|
| Food frequency questionnaire |
18 (78) |
| Food diary |
4 (17) |
| 24-hour recall |
1 (4) |
| Method of identifying dietary patterns |
|
| Principal component analysis |
15 (65) |
| Factor analysis |
5 (22) |
| Other† |
3 (13) |
| Number of dietary patterns: median (IQR) |
4 (3–6) |
| Method of calculating diet score |
|
| Weighted sum score |
12 (52) |
| Standardised sum score |
3 (13) |
| Other |
3 (13) |
| Not clear/not stated |
5 (22) |
| Outcomes reported |
|
| Bone density |
16 (70) |
| Fracture |
6 (26) |
| Both bone density and fracture |
1 (4) |
| Percentage of quality score: mean (SD) |
81.5 (7.3) |
*Values are n (%) unless otherwise specified
†Cluster analysis, reduced rank regression or mixture of methods
IQR, interquartile range; SD, standard deviation |
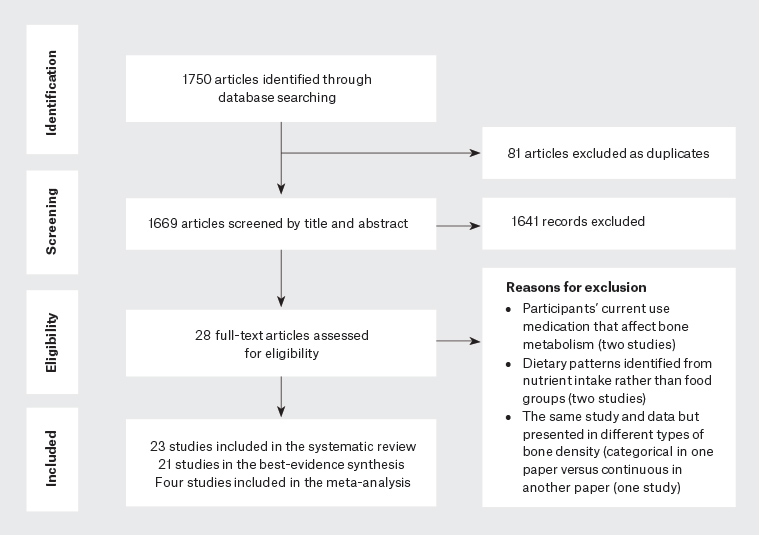
Figure 1. Flowchart of studies included in the systematic review and meta-analysis
Bone density outcomes
The results of individual studies are summarised in Table 2. For each bone density outcome, the evidence for associations with both ‘healthy’ and ‘Western’ patterns was conflicting. ‘Healthy’ dietary pattern score was positively associated with BMD in some studies (hip,24 lumbar spine,22,24 femoral neck,26,34 forearm15,16 and total body23) but not others (hip,18,20 lumbar spine,3,18–21 femoral neck,3,18,19,21,22 forearm,29 total body24 and TBBMC20).In two studies (lumbar spine23 and femoral neck15) there were positive associations of ‘healthy’ pattern score with BMD in men but no association in women. There were no negative associations between ‘healthy’ pattern score and any bone density outcomes. ‘Western’ dietary pattern score was negatively associated with BMD in some studies (hip,24 lumbar spine,3,19,24 femoral neck,19,26,34 forearm29 and total body bone mineral density [TBBMD]24) and TBBMC20 but had no associations in others (hip,20 lumbar spine,20–23 femoral neck,3,15,21,22 forearm,15,16 and TBBMD23,25 and TBBMC25). There were no beneficial associations of ‘Western’ pattern scores with bone density outcomes.
| Table 2. Associations between healthy-type and Western-type dietary patterns and bone density at different sites in individual studies |
| Author, year |
Findings* |
| ‘Healthy’ dietary pattern |
‘Western’ dietary pattern |
| Hip bone mineral density (g/cm2) |
| Fairweather-Tait, 201118 |
No association |
NA |
| McNaughton, 201120 |
No association |
No association |
Denova-Gutiérrez, 201624
|
+ve for low hip BMD† (OR 0.71; 95% CI: 0.44. 0.97 for highest vs lowest score quintile) |
−ve for low hip BMD† (OR 1.91; 95% CI: 1.19, 3.04 for highest vs lowest score quintile) |
| Lumbar spine bone mineral density (g/cm2) |
| Fairweather-Tait, 201118 |
No association |
NA |
| Hardcastle, 201119 |
No association
|
−ve (β −0.008; 95% CI: −0.013, −0.003 per unit score) |
| McNaughton, 201120 |
No association |
No association |
| Karamati, 20123 |
No association |
−ve for BMD below median (OR 2.29; 95% CI: 1.05, 4.96 for those with higher vs lower score) |
| Whittle, 201221 |
No association |
No association |
Shin, 201322
|
+ve for osteoporosis at lumbar spine (OR 0.47; 95% CI: 0.34, 0.65 for highest vs lowest quintile of score) |
No association |
Shin, 201523
|
+ve (β 0.016; 95% CI: 0.005, 0.027 per unit score in men)
No association in women |
No association
|
Denova-Gutiérrez, 201624
|
+ve for low lumbar spine BMD† (OR 0.80; 95% CI: 0.68, 0.94 for highest vs lowest quintile) |
−ve for low lumbar spine BMDb (OR 1.61; 95% CI: 1.06, 2.45 for highest vs lowest quintile) |
| Femoral neck bone mineral density (g/cm2) |
| Tucker, 200215 |
+ve for men: femoral neck BMD greatest in ‘fruit, vegetable & cereal’ group‡
No association in women |
No association |
| Langsetmo, 201026 |
+ve for men age 25−49 years (β 0.012; 95% CI: 0.002, 0.022 per SD score)
Results for other groups not reported |
−ve for men age 50+ years and postmenopausal women (β 0.009 [95% CI: 0.002, 0.016] and 0.004 [95% CI: 0.000, 0.008] per SD score)
Others not reported |
| Fairweather-Tait, 201118 |
No association |
NA |
| Hardcastle, 201119 |
No association
|
−ve (β −0.009; 95% CI : −0.013, −0.004 per unit score) |
| Karamati, 20123 |
No association |
No association |
| Whittle, 201221 |
No association |
No association |
| Shin, 201322 |
No association |
No association |
| De Jonge, 201634 |
+ve (β 0.06; 95% CI: 0.03, 0.08 per SD score) |
−ve (β −0.03; 95% CI: −0.06, −0.01 per SD score) |
| Total body bone mineral density (g/cm2) |
Shin, 201523
|
+ve (β 0.017 [95% CI: 0.008, 0.027] and 0.007 [95% CI: 0.000, 0.015]) per unit score in men and women respectively |
No association |
Denova-Gutiérrez, 201624
|
No association |
−ve for low TBBMD† (OR 1.74; 95% CI: 1.10, 2.76 for highest vs lowest quintile of score) |
Melaku, 201725
|
+ve (β 0.027; 95% CI: 0.001, 0.043 per tertile score by RRR)
No association using PCA or PLS |
No association |
| Forearm bone mineral density (g/cm2) |
| Tucker, 200215 |
BMD higher in a ‘fruits, vegetable and cereal’ group‡ (except alcohol group) in men and women |
No association |
| Okubo, 200616 |
+ve (mean of 0.498 vs 0.476 in top vs bottom quintile, P <0.05) |
No association |
Park, 201229
|
No association |
−ve for osteoporosis (RR 1.46; 95% CI: 1.02, 2.10 for highest vs lowest quintile) |
| Total body bone mineral content (g) |
| McNaughton, 201120 |
No association |
TBBMC (β −15.37 per quintile of score; 95% CI: −27.41, −3.34) |
| Melaku, 201725 |
+ve (β 69.65; 95% CI: 16.67, 122.63 per tertile score by RRR)
No association using PCA or PLS |
No association |
*+ve means beneficial association, −ve means detrimental association
†Low defined as ≤−1.0 T-score
‡Compared with other groups (‘meat, dairy and bread’, ‘meat and baked products’, sweet baked products’, alcohol and candy groups)
β, beta coefficient; BMC, bone mineral content; BMD, bone mineral density; CI, confidence interval; NA, not applicable; OR, odds ratio; PCA, principal component analysis; PLS, partial least-squares; RR, relative risk; RRR, reduced-rank regression methods of deriving patterns; SD, standard deviation; TBBMD, total body bone mineral density |
Fractures
Seven studies reported fracture outcomes.27,28,30,32,33,35,36 Over 70% of studies reporting fracture outcomes focused on older adults27,28,32,33,36 and over half reported results for both sexes combined.27,30,33,36 The available data made meta-analysis possible only for associations between lowest versus highest categories (tertiles, quartiles or quintiles) of ‘healthy’ dietary pattern score and hip fracture.30,32,35,36 There was a reduced risk of hip fracture with higher ‘healthy’ dietary pattern scores (RR 0.73; 95% CI: 0.56, 0.96; Figure 2). Heterogeneity was high (I2 = 95%, P <0.05). Heterogeneity was reduced and the effect size larger in those studies in which fracture was ascertained from medical records (RR 0.64; 95% CI: 0.56, 0.73; I2 = 67%).
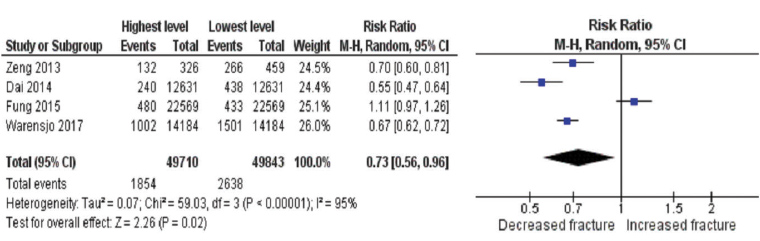
Figure 2. Forest plot of the association between healthy dietary pattern and hip fracture
Best-evidence synthesis was performed to examine associations of ‘Western’ pattern with hip and total fracture, and ‘healthy’ pattern with total fracture. Findings of these individual studies are given in Appendix 4. There was conflicting evidence for detrimental associations between ‘Western’ dietary pattern and both hip and total fracture and a beneficial association between ‘healthy’ pattern and total fracture. ‘Western’ pattern score was associated with higher hip fracture risk in three studies33,35,36 but there were no associations in two other studies.30,32 Similarly, one study33 reported a detrimental association between the ‘Western’ pattern and total fracture while there were no associations in two other studies.27,28 The risk of total fracture was lower with higher ‘healthy’ dietary pattern score in one study33 while another study reported mixed results with a reduced risk in women but no association in men.28 There were no detrimental associations between a ‘healthy’ pattern score and fracture, or beneficial associations between the ‘Western’ pattern and fracture in any study.
Discussion
This systematic review provides a robust site-specific synthesis of the evidence for the associations of empirically derived dietary patterns and site-specific bone density and fracture outcomes. Best-evidence synthesis was performed for most outcomes as meta-analysis was not possible. This assessed evidence supporting beneficial effects of a ‘healthy’ diet on bone density and detrimental effects of a ‘Western’ diet on both bone density and fracture outcomes as conflicting. However, studies consistently failed to demonstrate any detrimental effect of a ‘healthy’ pattern, nor any beneficial effects of a ‘Western’ pattern on bone outcomes. The meta-analysis demonstrated a beneficial association between ‘healthy’ dietary patterns and hip fracture in adults. These results suggest that having a ‘healthy’ diet and avoiding a ‘Western’ dietary pattern may be beneficial and is unlikely to be detrimental for bone health overall, and importantly, having a ‘healthy’ dietary pattern could help reduce the risk of hip fracture. In the absence of randomised controlled trials (RCTs), this observational evidence suggests that it is reasonable to incorporate dietary advice to consume a diet high in fruits, vegetables, nuts, fish, wholegrain and legumes and low in red meats, processed meats, fats, sweets, takeaway foods and soft drinks into recommendations for promoting bone health, particularly given the known wide-ranging health benefits of improving diet quality for preventing and managing a range of chronic diseases.
The present study has important differences from previous systematic reviews. Unlike the review of Denova-Gutiérrez,9 it examines bone outcomes separately for different sites. This is important as fracture risk factors7 and the costs and sequelae of fractures differ for different sites.8 Site-specific data are necessary to assess the potential clinical, public health and health economic37 benefits of any potential intervention. Denova-Gutiérrez et al reported a lower risk of fracture (the site[s] of which were not specified) in the highest compared to lowest categories of ‘healthy’ dietary pattern (odds ratio [OR] 0.81; 95% CI: 0.69, 0.95) in men, but no effect in women.9 This contrasts with the present data for hip fracture, which suggests that a ‘healthy’ diet may be important in both sexes for the prevention of this costly and damaging major osteoporotic fracture. Another systematic review10 only included studies that reported estimates of the risk of being in a ‘low’ BMD category, rather than associations with BMD as a continuous outcome. This meant that evidence from many studies included in the present review was not considered. Arguably, the review approach used in the present study could be considered to provide a more comprehensive assessment of the available evidence. That second review10 reported a risk reduction (OR 0.82) for ‘low’ BMD when pooling data from all sites but inconsistent associations when analysing site-specific BMD, and subgroups by age and sex.
The findings of the current study that are most relevant to clinical practice are that a ‘healthy’ dietary pattern was associated with reduced risk of hip fracture, by as much as 36%, and that studies of bone density outcomes were consistent in that none reported detrimental associations of a ‘healthy’ pattern nor a beneficial association of a ‘Western’ pattern with BMD. The association with hip fracture is of a substantial magnitude, of both clinical and public health importance. It is comparable with the effect size observed in a recent RCT of zoledronate for hip fracture among older women with osteopaenia (hazard ratio [HR] 0.66; 95% CI: 0.27, 1.16, when compared with placebo)38 and to the effects of other bisphosphonates.39 Results of the present study suggest that promoting the consumption of a diet high in fruits, vegetables, nuts, fish, wholegrains and legumes and low in red meats, processed meats, fats, sweets, takeaway foods and soft drinks could be incorporated into guidelines for promoting bone health in adults. Importantly, there are potential benefits of such dietary changes for many chronic diseases and this advice is already embedded in dietary recommendations around the world, including Australia.40 The evidence in the present review comes solely from observational studies, and thus must be interpreted with caution, and RCTs of interventions to improve dietary patterns are needed to definitively assess the effects of dietary changes on bone health. However, RCTs of behavioural interventions with fracture outcomes will be large and logistically challenging, as seen in one of the few such trials for cardiovascular outcomes,41 so this is likely to remain an evidence gap for some time. Given the wide-ranging potential health benefits of the proposed advice, the evidence suggesting there could be substantial potential benefits of a ‘healthy’ dietary pattern for hip fracture, and the lack of evidence of any detrimental impacts, it seems reasonable and warranted to implement this advice for bone health now.
The major strength of this study is the use of meta-analysis where it was possible and a structured best-evidence synthesis approach otherwise, which together result in assembling the strongest and most comprehensive evidence from the available data. This systematic review also has limitations. A major limitation is the cross-sectional nature of most available data, which precludes attribution of causation. To accurately determine the true effect of improving diet on bone outcomes, better evidence from longitudinal studies and RCTs is needed. The lack of comparability in analytical approaches and measurement of outcomes across studies limited the meta-analysis to the single outcome of hip fracture, and to two categories of dietary patterns. The evidence for all the outcomes assessed by best-evidence synthesis was also conflicting. This is not surprising, given the diversity in study design, methods and populations. Nonetheless, it is reassuring that there is consistency with regards to the lack of any evidence for possible detrimental bone outcomes from recommending either promoting a ‘healthy’ diet pattern or advising avoidance of a ‘Western’ pattern. Finally, the formal search was completed in 2017. However, the researchers have identified only four other studies reporting dietary patterns similar to those for which data synthesis was performed published since then and they only report bone density outcomes. Like those in the present review, their results are inconsistent42–45 and they do not materially affect the present results or conclusions.
In conclusion, the current observational evidence suggests that it is reasonable to incorporate existing population health recommendations for a ‘healthy’ diet into recommendations for promoting bone health, especially given the known wide-ranging health benefits of improving diet quality for the prevention and management of a range of chronic diseases.