Case
A man aged 50 years was referred to a multidisciplinary dermatology clinic. He reported a three-year history of an asymptomatic scalp lesion that had persisted after three episodes of cryotherapy for a presumed diagnosis of solar keratoses. He also reported a history of basal cell carcinoma (BCC) on his trunk, as well as solar keratoses. He was otherwise well and taking no medications. He was a Caucasian indoor worker with a long history of recreational sun exposure and no personal or family history of melanoma.
Examination of the scalp revealed an ulcerated papule with a diameter of 9 mm (Figure 1). Dermoscopy (Figure 2) showed ulceration and some slight scaling. There was no visible pigmentation, and there were no vessels.
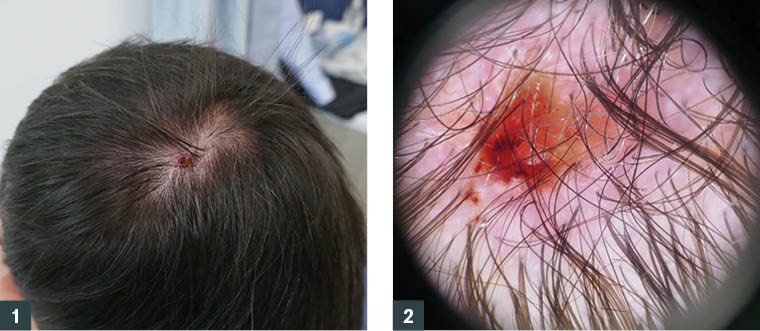
Figure 1. Ulcerated papule scalp lesion
Figure 2. Dermoscopy of the lesion
Question 1
What is the differential diagnosis?
Question 2
What other examinations and investigations should be carried out?
Answer 1
The most likely diagnoses are a BCC or squamous cell carcinoma (SCC). However, repeated cryotherapy and the presence of ulceration may modify diagnostic features. Therefore, a broad range of diagnoses needs to be considered. Refer to Table 1 for differentials for an ulcerated papule scalp lesion.
| Table 1. Differential diagnoses for an ulcerated papule scalp lesion |
| Condition |
Clinical features |
Dermoscopic features |
| Basal cell carcinoma (BCC)9,10 |
Nodular variant is most common, with pearly white papule with overlaying telangiectasia. Frequently ulcerates, bleeds and becomes crusted in the centre. Other variants may be pigmented, flat or circumscribed red scaling plaque.
|
- Linear and arborising telangiectasia
- Leaflike structures
- Blue–grey ovoid nests and globules
- Spoke wheel areas
- Focal ulceration and crystalline structures
|
| Squamous cell carcinoma (SCC)11,12 |
May present as a poorly defined erythematous scaling papule or plaque. Can produce a keratin horn. Ulceration is common. Pain and tenderness is common. May develop over weeks to months. |
- Keratinisation with white structureless areas
- White perifollicular circles
- Blood spots
- Variable blood vessels
|
| Merkel cell carcinoma13,14 |
Typically a fast-growing red nodule with a shiny surface and overlaying telangiectasia. |
- Linear, irregular and polymorphous vessels
- Poorly focused vessels
- Milky pink/white areas
- Structureless areas
- Architectural disorder
- Pigmented structures typically absent
|
| Atypical fibroxanthoma15,16 |
Typically occurring on head and neck of the elderly and occurring as a solitary red dome-shaped nodule that may ulcerate, crust and bleed. |
- Often presents similarly to a BCC or SCC
- Red and white structureless areas
- Irregular linear vessels
- Absence of yellowish-white opaque scales, hairpin vessels and arborising vessels
|
Inflamed seborrheic
keratosis17–19
|
Flat or raised with variable appearance, often a waxy or ‘stuck on’ appearance. Sharply demarcated border, and may have the ‘wobble sign’. |
- Milia-like cysts
- Comedo-like openings
- Fissures and ridges
- Network-like structures
- Cerebriform pattern
- Fat fingers
- Sharply demarcated borders
- Typical hairpin blood vessels
|
Inflamed actinic keratoses20,21
|
Initially a poorly defined redness, which then develops keratotic scale. Varied appearance but can develop a flat or thickened papule/plaque with scale/warty surface.
|
- Irregular shape
- Surface scale
- Erythematous
- Dotted vessels
- Short shiny streaks
- Rosettes
|
Pyogenic
granuloma22
|
Grows rapidly and first appears as a painless, shiny red lump. Typically painless but bleeds easily and may ulcerate and form a crusted sore.
|
- Reddish homogenous areas
- White rail lines
- White collarette
- Varied vascular structures
|
| Amelanotic melanoma23,24 |
Varied presentation and may begin as asymmetrical macular lesions that may be uniformly pink or red and may have a faint light tan, brown or grey pigmentation at the periphery. The borders may be well or poorly defined.
|
- Irregular dots or globules
- Irregular pigmentation
- Polymorphous vascular patterns including:
- milky-red areas
- reticular depigmentation
- irregular dotted and/or linear vessels
- white lines
|
Answer 2
A full skin check needs to be performed. If there is a high burden of skin malignancy, treatment by a plastic surgeon in one operating session may be preferable.
As SCC and melanoma are a possibility, the patient should be clinically examined for evidence of lymphatic dissemination. The lesion also requires biopsy as this will guide further management.
Case continued
A full skin check revealed 15 BCCs scattered over his face, trunk and limbs.
An 8 mm × 6 mm × 1 mm shave biopsy of the scalp lesion revealed an ulcerated solid BCC.
The excised scalp lesion was found to be an infiltrative BCC with perineural invasion (PNI) of a nerve 0.1 mm in diameter at the base of the tumour near the junction between the dermis and subcutis. The identifiable tumour was approximately 5 mm from the nearest side margin of excision and 3 mm from the deep margin. The affected nerve appeared to involve an inflamed fibrous tract extending to the 3 o’clock margin.
The other lesions were all either excised or curetted and showed a mix of superficial and solid BCCs.
Question 3
Does this lesion need further treatment? If so, what options would you consider?
Answer 3
This was a recurrent BCC with an aggressive growth pattern showing PNI and possible margin involvement. The presence of the inflamed fibrous tract extending to a margin meant further treatment was needed. An aggressive growth pattern with PNI is related to heightened risk of locoregional recurrence and poorer prognosis regardless of clear margins on histology.1 PNI is seen in 2–6% of cutaneous head and neck SCC and BCC, and up to 10% of BCC.2,3
Adverse features with PNI include:
- large nerve size >0.1 mm
- multiple nerves involved
- extension of PNI outside the tumour
- clinically evident PNI (paraesthesia, numbness, loss of motor function).4
Low-risk features with PNI include:5
- nil motor/sensory changes
- intratumoural PNI
- no large nerve involvement (>0.1 mm) on histology
- no invasion beyond the dermis
- no diffuse intratumoural spread.
Options for further treatment include:
- wide excision
- Mohs micrographic surgery or other form of margin control surgery
- adjuvant radiation treatment, either as definitive treatment or after re-excision depending on the outcome.
Case continued
The scar was re-excised, producing a specimen 74 mm × 12 mm × 9 mm ellipse with a 7 mm margin. The defect could be sufficiently closed without the need for a flap or graft, and the surgeon used staples. Histology was reported as showing scar only with no viable BCC identified, nor evidence of residual perineural disease. Margins appeared well clear around the site.
As the patient’s PNI was classified as low risk, radiotherapy was not used, as per discussion with radiation oncology.
Question 4
What were the indications for wide excision without margin control in the context of this patient?
Answer 4
A simple wide excision was indicated as there was only one area of possible margin involvement by the perineural spread in one nerve 0.1 mm in size and without definite extension beyond the tumour; therefore, the lesion was classified as low risk.5 The nerve involvement was asymptomatic and thus is defined as an incidental finding, which is a low-risk feature. PNI that has associated sensory/motor changes and/or positive imaging is likely to require adjuvant radiotherapy.
Using staples rather than sutures lessens the risk of tissue and hair follicle strangulation.6 Figure-of-eight pulley stitches (used alone or with staples) are another good option on the highly vascularised scalp.7 Mohs surgery was unlikely to have conferred a higher cure rate, so while it is a good choice for cosmetically and anatomically sensitive areas, and where tissue preservation may be of concern, it is a labour-intensive and costly procedure and was deemed unnecessary in this instance.
Some indications for Mohs surgery are outlined in Table 2.8
| Table 2. Indications for Mohs surgery |
| Tumour feature |
Location |
| Tumour in high-risk area |
High-risk areas (central face, nose, lips, eyelids, eyebrows, periorbital skin, chin, mandible, ears, preauricular and postauricular areas, temples, hands, feet)
Site of previous radiation therapy |
| Tumour size ≥10 mm |
Areas of the face, neck or scalp not mentioned above |
| Tumour size ≥20 mm |
Trunk or limbs |
| Tumours with aggressive pathological features |
Anywhere |
| Recurrent tumour |
Anywhere |
| Tumour with poorly defined borders |
Anywhere |
| Tumour occurring in patient with immunocompromise |
Anywhere |
Key points
- Cryotherapy may obscure clinical and dermoscopic features; if a lesion does not respond as expected, a biopsy is recommended.
- A full skin check should always be performed when a patient presents with an index lesion, as solar-induced skin malignancy often occurs in multiples.
- In BCC management, aggressive features and/or PNI warrant multidisciplinary care (including radio-oncology), even if the specimen has clear margins.