Chronic airway diseases are highly prevalent in Australia, with asthma affecting more than one in 10 people, and chronic obstructive pulmonary disease (COPD) affecting approximately 7.5% of people over the age of 40 years.1,2 The mainstay of treatment for these conditions is the daily delivery of medicines from inhaler devices. Australians therefore consume millions of inhalers annually.3
The manufacture, transportation and disposal of all inhalers uses energy, causes some pollution with greenhouse gases and creates material waste. However, there are additional pollution burdens from pressurised metered-dose inhalers (pMDIs) because of the properties of the propellant gases inside the inhaler canister.
Previously, pMDIs used chlorofluorocarbon (CFC) propellants, which contributed to both the depletion of the ozone layer and global warming. The Montreal Protocol, signed in 1987, aimed to restore the ozone layer by phasing out CFCs from diverse industrial applications, including respiratory inhalers. For many years, pMDIs in Australia have been CFC-free, with hydrofluorocarbons (HFCs) used as alternative propellants. Unfortunately, while ozone safe, HFCs are also potent greenhouse gases, with an effect thousands of times greater than the equivalent volume of carbon dioxide (CO2). The Kigali Amendment to the protocol, effective from 2019, seeks to reduce the emissions of HFCs.4 This has been accepted or ratified by more than 130 states, but it has not yet led to changes in the HFCs used in commercially available pMDIs.
The contribution of inhaler propellants to climate change is especially a concern given that pMDIs have long been the preferred option for prescribers in Australia. For example, in 2021, pMDI versions of fluticasone/salmeterol were dispensed approximately twice as commonly as the dry powder inhaler (DPI) versions.3
Figure 1 shows estimates of the relative amount of global warming potential (in CO2 equivalent [CO2e]) produced at each stage of life of three common inhalers.5 The figure highlights the major contribution from propellant released with dosing, and then when inhalers leak remaining propellant after disposal.
To provide some perspective, the reduction in carbon footprint achieved by a person switching from regular pMDI use to DPI use (approximately 420 kg CO2e annually) is similar to them switching their petrol car to a hybrid (approximately 500 kg CO2e annually) or becoming vegetarian (approximately 660 CO2e annually)5,6 – a substantial proportion of individual carbon footprints.
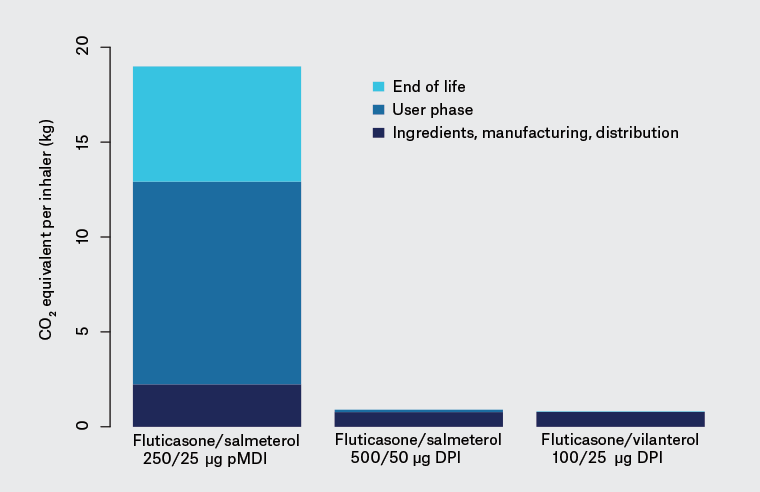
Figure 1. Relative equivalent amount of carbon dioxide per 30-day inhaled treatment with one of the inhalers shown (ie per inhaler, not total population use)5
CO2, carbon dioxide; DPI, dry powder inhaler; pMDI, pressurised metered-dose inhaler
What can general practitioners do?
Accurate diagnosis
It is important that diagnosis comes before management. Accordingly, the first thing general practitioners (GPs) can do to reduce the environmental impact of inhaled therapies is to make an accurate diagnosis. Recent research from a large community sample in Canada has found that approximately one-third of people diagnosed with asthma, and approximately half of people diagnosed with COPD, cannot have their diagnoses proven after careful evaluation, even after withdrawal of treatment.7,8 It is important to establish diagnoses as carefully as possible to avoid overtreatment and incorrect treatment.
In addition to history and physical examination, a diagnosis of asthma or COPD ideally involves objective testing. Access to lung function testing is more difficult than ever during the COVID-19 pandemic. At present, new cleaning regimens slow the work of respiratory testing, and health professionals are encouraged to avoid spirometry in patients with viral-type symptoms.9 The latter especially challenges the detection of asthma in patients who only have symptoms when they seem virally unwell. Ideally, health systems would enable referral for more detailed evaluation, such as challenge tests, before committing patients with apparent chronic airway disease to long-term daily inhaled therapy.
Good disease control
Poor asthma control often leads patients to overuse short-acting β-agonists (SABAs). This is especially the case in Australia, where SABAs are easily available over the counter, and significant proportions of people with asthma report a combination of frequent SABA use and little or no preventer medicine.10,11
Australian GPs will be familiar with the message that good asthma care, including the use of inhaled corticosteroids, improves symptoms and reduces the risk of severe exacerbations, hospitalisations, long-term lung function decline and death. Similar positive interventions also exist for COPD (Box 1). However, the idea that good asthma control is also a positive thing for the environment may be unfamiliar. This is because good disease control helps to prevent excessive use of SABA inhalers, most of which are of the polluting pMDI variety (including all salbutamol inhalers in Australia). This is a win–win for our patients and our planet.
| Box 1. Examples of evidence-based interventions that improve control or decrease exacerbations and thus reduce pressurised metered-dose inhaler consumption |
Potential actions in a primary care consultation
- Smoking cessation advice support
- Supporting with weight loss for people with obesity
- Ensuring good inhaler technique
- Checking and addressing concordance
- Advising about exacerbating factors, such as occupational allergens or wood burning stove at home
- Treating comorbidities such as rhinitis
- Including inhaled corticosteroids in asthma treatment (nearly all people with asthma)
- Switching to single maintenance and reliever therapy (moderate-to-severe asthma)
- Adding a long-acting antimuscarinic (severe asthma)
- Recommending handheld fan (COPD)
- Providing immunisation, especially influenza and pneumococcal vaccines for people with COPD
Actions that might require respiratory specialist input
- Pulmonary rehabilitation referral
- Breathing retraining exercises (asthma)
- Use of opiates for breathlessness (COPD)
Respiratory specialist interventions
- Systematic multidisciplinary assessment
- Specialist medicines such as monoclonal antibodies (for some patients)
- Management of ‘asthma mimics’, such as inducible laryngeal obstruction, if present
- Interventions such as bronchial thermoplasty or lung volume reduction (for some patients)
|
| COPD, chronic obstructive pulmonary disease |
Choosing inhalers other than metered‑dose inhalers
A core aspect of reducing the environmental impact of inhaled therapy is to prescribe inhalers other than pMDIs. There is now a wide array of DPIs on the Australian market, including Turbuhaler, Accuhaler, Ellipta, Spiromax, Handihaler, Breezhaler, Zonda and Genuair devices. A soft mist inhaler device called Respimat is also available.12 All of these inhalers are free of the HFC propellant gases that make MDIs so polluting.
DPIs are appropriate for many patients, though some exceptions apply. Because DPIs rely on inspiratory flow being sufficient to disaggregate and sweep the dry powder into the lungs, they are not recommended in children under the age of six years or people with poor inspiratory flow.13 Formal measurement of inspiratory flow is not usually available in general practice, but a practical rule of thumb is to see if the patient, after exhaling, can take a quick deep breath in less than three seconds. If so, inspiratory ability is considered sufficient for a DPI.14 Most adolescents and adults will meet this standard.
In appropriate patients, DPIs are at least as effective as pMDIs for clinical outcomes in asthma. There is level 1 evidence showing no difference in randomised trials between these inhaler types for both β-agonists and inhaled corticosteroids.15,16 Some real-world evidence finds DPIs superior, perhaps because of their simplicity and ability to be used without a spacer.17 GPs can therefore be confident in reassuring most patients that DPIs are likely to work at least as well as pMDIs. Indeed, a recent trial in England found that asthma control improved in patients moved from pMDIs to a DPI, while inhaler carbon footprint was reduced by more than half.18 Guidance from the National Institute for Health Care and Excellence in the UK includes environmental considerations in their inhaler decision aid.19
Table 1 compares the MDI and non-MDI options at each step of the current Australian Asthma Handbook adult asthma guidelines.20 As this shows, there are non-MDI inhaler options at every treatment step in the current Australian guidelines.
| Table 1. Greener versus more polluting options for each guideline step and strategy* |
| Step |
Strategy |
Dry powder/soft mist (greener) options |
Metered-dose inhaler (more polluting) options |
| 5 |
Add-on specialised treatment – refer to non-GP specialist |
May include LAMA:
tiotropium soft mist inhaler
(with separate ICS/LABA inhaler)
or in combination:
fluticasone/umeclidinium/vilanterol DPI
indacaterol/glycopyrronium/mometasone DPI |
|
| 4 |
ICS/formoterol maintenance and reliever therapy (medium-dose inhaler as maintenance and
low-dose inhaler as reliever) |
Budesonide/formoterol DPI |
Budesonide/formoterol pMDI
Beclometasone/formoterol pMDI |
| Regular maintenance ICS/LABA (medium-to-high dose) + SABA reliever as needed |
Budesonide/formoterol DPI
Fluticasone propionate/salmeterol DPI
Fluticasone furoate/vilanterol DPI
Mometasone/indacaterol DPI
SABA option as per step 1 |
Budesonide/formoterol pMDI
Fluticasone propionate/salmeterol pMDIs
Beclometasone/formoterol pMDI
Fluticasone propionate/formoterol pMDI
SABA options as per step 1 |
| 3 |
ICS/formoterol maintenance and reliever therapy (low dose; single low-dose inhaler as both maintenance and reliever) |
Budesonide/formoterol DPI |
Budesonide/formoterol pMDI 100/3 or 50/3 μg
Beclometasone/formoterol pMDI 100/6 μg |
| Regular maintenance ICS/LABA (low dose) + SABA reliever as needed |
Budesonide/formoterol DPI
Fluticasone propionate/salmeterol DPI
Mometasone/indacaterol DPI
SABA option as per step 1 |
Budesonide/formoterol pMDI
Fluticasone propionate/salmeterol pMDI
Beclometasone/formoterol pMDI
Fluticasone propionate/formoterol pMDI
SABA options as per step 1 |
| 2 |
Regular maintenance ICS (low dose) + SABA reliever as needed |
Budesonide DPI 200–400 μg/day
SABA option as per step 1 |
Beclometasone 100–200 μg/day pMDI
Ciclesonide 80–160 μg/day pMDI
Fluticasone propionate pMDI 100–200 μg/day
SABA options as per step 1 |
| Budesonide/formoterol (low dose) as needed |
Budesonide/formoterol DPI
(200/6 μg, one inhalation per dose) |
Budesonide/formoterol pMDI
(100/3 μg, two puffs per dose) |
| 1 |
As-needed SABA alone
(Appropriate for very few patients – only if symptoms are less frequent than twice/month and there are no flare-up risk factors) |
Terbutaline DPI (PBS restrictions apply) |
Salbutamol pMDI |
*This table categorises current Australian Asthma Handbook recommendations20 according to the pollution potential of inhaler devices.
DPI, dry powder inhaler; ICS, inhaled corticosteroid; GP, general practitioner; LABA, long-acting β-agonist; LAMA; long-acting muscarinic antagonist; PBS, Pharmaceutical Benefits Scheme; pMDI, pressurised metered-dose inhaler; SABA, short-acting β-agonist |
One barrier to using greener inhalers in Australia is the restricted Pharmaceutical Benefits Scheme (PBS) availability of terbutaline, the sole SABA available in a DPI. Consequently, terbutaline is modestly more expensive than salbutamol for many patients. However, it should be noted that as-needed budesonide/formoterol is a reliever option in Australian and international asthma guidelines, and this is available as a DPI on the PBS.
Terbutaline aside, DPIs and pMDIs have similar costs to patients,21 so cost need not usually be a barrier to change.
In Figure 2 we offer our suggested ‘CACTUS’ model for the inhaler prescribing process in adults and older children, which is inspired by an existing guideline.14 Decision making incorporates the patient’s ability to inhale and use the device, their preferences and a consideration of costs. The model includes the importance of demonstrating the device and checking for patient understanding and inhaler technique.
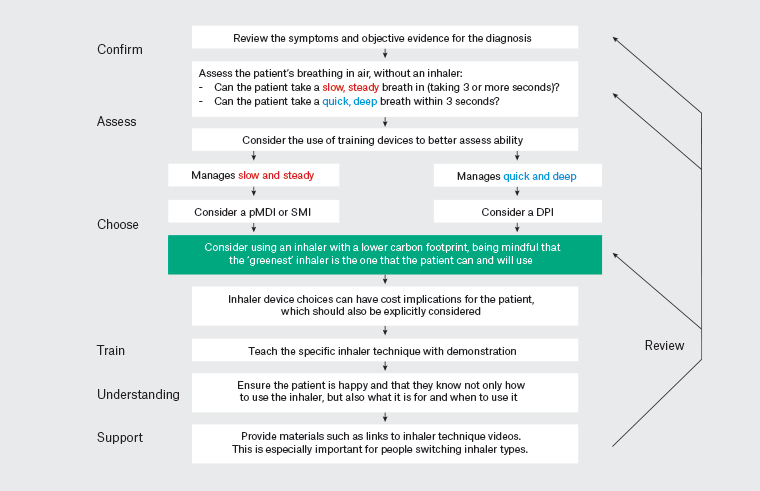
Figure 2. The CACTUS model for the inhaler prescribing process for people over the age of six years14 Click here to enlarge.
Other ways to reduce waste
Apart from choosing inhalers that do not emit polluting propellant gases, there are other ways to reduce waste from respiratory inhalers.
One is to choose medication regimens that use fewer doses and consequently fewer inhalers, reducing plastic waste. For example, budesonide 400 μg taken once daily has equivalent clinical outcomes to the more traditional dosage of 200 μg twice daily.22 This prescribing choice can halve the amount of plastic waste generated and reduce cost and inconvenience to patients.
Another example is the use of budesonide/formoterol 200/6 μg DPIs as an as-needed medication for mild asthma (step 2 in Table 1). On average, people using this management strategy use substantially fewer doses of medication than people prescribed a traditional regular inhaled corticosteroid regimen.23
For patients who are best served by a pMDI, pollution and waste can be minimised by using the device until it is empty. Dose counters built into inhalers can help with this.
Some inhalers are partly reusable, such as Handihaler and Respimat devices. We hope to see more options for reuse in the future.
We look forward to the prospect of using newer HFC propellants that have similar pharmacological properties but a much lower greenhouse impact. The active development of inhalers using these has been accelerated following European Union legislation, and major pharmaceutical companies have progressed toward widespread implementation in the next few years.24,25
We also look forward to further innovations in the delivery of recycling of inhalers. Recycling of plastic and metal from inhaler devices is possible and has been trialled in several countries.26 However, unlike the plastic generated by other industries (eg soft drink bottles), recycling of inhalers has been left to the manufacturer. We hope for sustainable industry–government initiatives in this area in the future, although with Australia’s relatively poor track record of environmental leadership, our expectations are low.
Conclusion
Climate change has profound implications for human health.27 A safe response requires all sectors of society to mitigate emissions as quickly as possible.
Australia’s health system is responsible for 7% of the national carbon footprint.28 There will be many challenges to reducing our health system’s impact on our climate, but thinking carefully about respiratory inhaler prescribing is one of the easiest places to start.
Accurate diagnoses, quality disease management and careful consideration of inhaler types are all important steps in reducing the impact of inhaled respiratory therapies on the environment.
We believe that environmental considerations should be among the many factors weighed by doctors and patients in the prescribing process. For people with strong reasons to be using a pMDI, care should be taken not to burden patients with guilt. But for many other patients, discussing this topic may be an opportunity to further empower them to tread more lightly on the earth while also optimising their respiratory health.
Key points
- Respiratory inhalers contribute significantly to climate change, principally because the propellant gases in pMDIs are potent global warming gases.
- Doctors can reduce the climate impact of their prescribing by ensuring accurate diagnoses before prescribing, helping patients to optimise disease control and choosing inhalers other than pMDIs.
- DPIs achieve equivalent clinical benefits to pMDIs in most people with respiratory disease. Exceptions include children under the age of six years and people with unusually limited inspiratory flow.
- There are non-MDI inhaler options available at every treatment step in the current Australian guidelines.