In Australia as elsewhere, polypharmacy is increasing,1 attributed to an ageing population, its associated multimorbidities, expanding guidelines and patient demand.2 Long-term prescribing predominantly occurs in general practice due its central role:3 in 2017–18, Australian general practitioners (GPs) prescribed approximately 89% of all dispensed Pharmaceutical Benefits Scheme and Repatriation Pharmaceutical Benefits Scheme prescriptions.4 Collated GP national reports have estimated that medication-related harm occurred in approximately one in 10 patients in the preceding six months, with adverse reactions due to drug interactions and contraindications responsible for approximately 2.5% of these.5,6 When harm from inadequate monitoring is included, the estimation from a UK study was that 12% of primary care patients are affected by medication errors.7 Although the proportion of serious errors in primary care is low, given the overall number of prescriptions in absolute terms there is still potential for harm, with the consequences including hospitalisation.7 Across New South Wales (NSW) hospitals, annual medication-related admission rates doubled from 2001–02 to 2013–14, with ‘high-risk’ medicines – anticoagulants and opioids – the top two groups responsible.8
General practice is the central point for patients as they transfer to and from hospital and aged care, between specialists and other prescribers. These transitions of care are error-prone situations associated with harm,9 especially for patients at risk due to multiple health conditions, high-risk medicines and polypharmacy.7 One option to mitigate risks from polypharmacy in general practice is by planning a review of medicines in order to reduce or stop those that may be causing harm or are no longer beneficial,3 known as ‘deprescribing’. Deprescribing is not necessarily a new concept – it is considered an essential component of good prescribing practice.10,11 Engaging a clinical pharmacist in medication reviews and deprescribing in general practice has previously been proposed, especially for patients with complex regimens.3 Employing a non-dispensing general practice pharmacist (GPP) is a practical strategy to enable reviews, and such engagement is becoming more widespread as the scope of practice for pharmacists broadens internationally.12 A Royal Australian College of General Practitioners position statement recognises the potential role for GPPs in reducing overall prescribing and medication-related problems, but also recognises the need for more Australian-based research into the impact on health outcomes.13
A pilot study of a GPP that targeted patients at risk of medication-related harm has provided the opportunity to investigate the GPP’s influence on medication management, which includes, for this setting, initiation, ongoing review, monitoring and possible cessation, or deprescribing, of medicines.14 The aim of this study was to explore the influence of an embedded GPP on the medication management for patients at risk of medication-related harm, from the perspectives of GPs, general practice personnel, patients and carers who participated in this pilot study.
Methods
Context
This qualitative study forms part of the evaluation of a larger project that piloted a GPP model of care to improve management of patients at risk of medication-related harm, particularly from opioids. The pilot was conducted in a practice in regional NSW from September 2019 to May 2020, and included all 10 GPs, three practice nurses, a practice manager and referred patients who consented to GPP consultations (Table 1). The GPP was integrated into the practice workflow (Figure 1).
| Table 1. Steps in pilot general practice pharmacist model of care for patients at risk of medication-related harm |
| Steps |
Process |
Participants |
| 1. At-risk patient referred to GPP |
Criteria for at-risk patient referrals:
Patient:
- has had recent hospital discharge*
- has external prescribers*
- is transitioning to aged care*
- has a planned surgical procedure*
- is prescribed ≥1 regular opioid suitable for review
Consenting patients asked to bring all current prescription and non‑prescription medicines to consultation |
- GP
- Practice nurse
- Patient
|
2.
GPP preparation for patient consultation |
GPP review of indication for consultation and patient’s practice EMR:
- prior consultations and investigations
- medical conditions
- current and previous medications, allergies
- correspondence from other healthcare providers
|
- GPP
- If required: medical specialists, hospital prescribers, hospital or community pharmacists
|
3.
Patient–GPP consultation with reconciliation and review of all medicines† |
Pharmacist investigation to undertake medicines reconciliation and review:35
- Is there a current indication for the medication?
- Are the indication and dose appropriate, considering comorbidities and current clinical situation?
- Is the medication safe?
- What are the patient’s (and/or carer’s) views?
- Are medicines missing that the patient could be taking?
- Is the patient taking prescription, complementary or OTC medicines not documented in EMR?
|
|
| 4. GPP–GP clinical handover |
- Findings and recommendations documented in patient’s EMR
- Verbal handover to patient’s GP, if possible
- Record uploaded to patient’s MHR, if complete
|
|
5.
GP–patient (± carer) consultation ± GPP |
GP review of pharmacist’s notes and recommendations in patient’s EMR arising from medicines reconciliation and review:
- GPP present as required
- Decision making with patient
- Agreed medication regimen and plan for ongoing monitoring and review as required
|
|
*Plus at least one of: ≥5 regular medicines; ≥4 comorbidities; prescribed a high-risk medicine; chronic kidney disease
†Repeated from step 2, as required for ongoing management
EMR, electronic medical record; GP, general practitioner; GPP, general practice pharmacist; MHR, My Health Record; OTC, over-the-counter (medicines purchased by patient) |
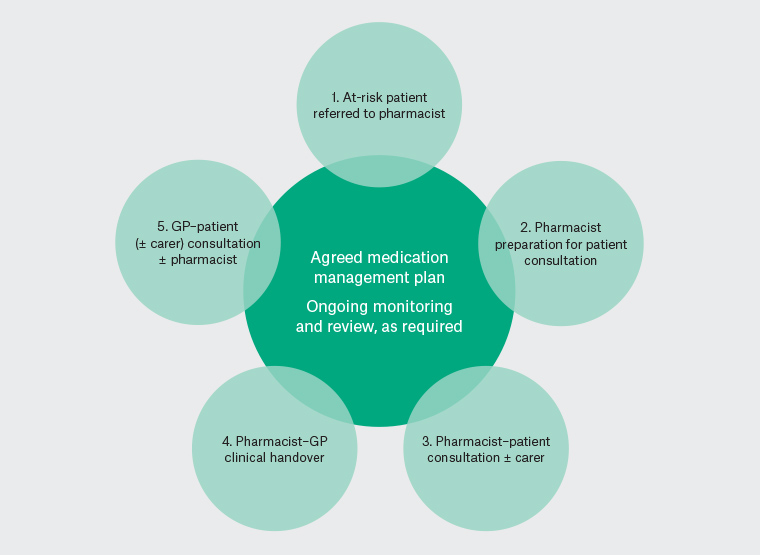
Figure 1. Pilot general practice pharmacist model of care for management for patients at risk of medication-related harm
GP, general practitioner
Qualitative approach
The article is guided by the Standards for reporting qualitative research criteria.15 Verbatim interview data relevant to medication management were obtained from semi-structured interviews with participants with lived experience of the pilot in order to investigate their perspectives. An iterative and inductive approach to thematic analysis was used, as described by Braun and Clarke.16
Sampling strategy
Sampling was purposive. All GPs, practice personnel and patients or their carers who participated were invited via email or telephone to be interviewed. Recruitment was facilitated by reception personnel, who forwarded details of consenting participants to interviewers. Arrangements thereafter were made between those parties. Due to social distancing requirements, consent was implied with response, confirmed at interview and documented.
Researcher characteristics and reflexivity
Participants were interviewed by two researchers. The first (JM), an experienced qualitative interviewer, researcher and pharmacist, conducted two interviews (GP01, GP02). The second (MYW), a medical student with a psychology and community pharmacy background, conducted the remainder. Prior to the interviews, neither had dealings with participants. Details of their professional roles were provided to interviewees if requested. Co-investigators not directly involved in data collection reviewed the data multiple times during analysis. The first author (MJ) was the pilot pharmacist.
Data collection methods and instruments
Semi-structured interviews, conducted by telephone, took place September–October 2020, four months after pilot completion. The interview schedule (Appendix 1), developed to address overall study aims, was used for all interviews. Participants were interviewed individually or, for some patients, with their carer. The interviews ranged from 10 to 35 minutes. Interviews were recorded on two devices, professionally transcribed verbatim and verified for integrity by two authors (MYW, MJ). Identifying details were redacted. Each participant had an individual code designated. Transcripts were not returned to participants for comment.
Data processing and analysis
Data collection ceased when all invited and consenting participants had been interviewed. This represented 100% of GPs, 75% of relevant practice personnel and all volunteering patients or carers; therefore, saturation was not sought. Data were coded independently by two authors (MYW, MJ) using NVivo 12 Plus and were explored using the six phases of thematic analysis.16 After data familiarisation, codes were separately identified, collaboratively refined and combined. Themes were developed inductively and represented contextual interpretations of participant data with supporting concordant or discordant quotations, as the approach taken to analysis was reflexive.17 Four authors (MJ, MYW, JM, TC) contributed to iteratively defining and refining the themes. Triangulation of data was performed to explore perspectives from separate participant types, enhancing interpretive rigour.18
Ethical review
The study was approved by the Health and Medical Human Research Ethics Committee of the University of Wollongong and Illawarra Shoalhaven Local Health District (approval no. 2019/259).
Results
Ten GPs, two practice nurses and the practice manager, five carers and 10 patients were interviewed (Table 2).
| Table 2. Characteristics of interview participants (n = 28) |
| Participant type |
Code |
Time working in general practice (years) |
Practice personnel
sex |
| General practitioner |
GP01 |
10 |
Female (F) |
| GP02 |
30 |
Male (M) |
| GP03 |
14 |
M |
| GP04 |
14 |
F |
| GP05 |
28 |
F |
| GP06 |
32 |
M |
| GP07 |
14 |
M |
| GP08 |
1 |
F |
| GP09 |
20 |
M |
| GP10 |
5 |
F |
| Practice personnel |
|
Time in role (years) |
|
| Practice manager |
PM |
10 |
F |
| Practice nurse |
PN01 |
8 |
F |
| Practice nurse |
PN02 |
26 |
F |
| Patients and carers |
|
Time as patient at this practice |
Patient sex (age in years) |
| Carer alone |
Carer01 |
45 years |
M (78) |
| Carer02 |
32 years |
F (91) |
| Carer03 |
20 years |
M (83) |
| Carer with patient |
Carer04 |
10 years |
M (87) |
| Carer05 |
3 years |
F (73) |
| Patient |
Pt01 |
10 years |
F (82) |
| Pt02 |
7 years |
F (81) |
| Pt03 |
10 years |
F (82) |
| Pt04* |
|
|
| Pt05 |
30 years |
F (81) |
| Pt06 |
7 years |
M (73) |
| Pt07 |
16 years |
M (75) |
| Pt08 |
10 years |
F (84) |
| Pt09 |
15 months |
F (69) |
| Pt10 |
10 months |
M (79) |
| Pt11 |
66 years |
F (87) |
| *Became ‘carer with patient’ early in course of interview (Carer04) |
Qualitative analysis
Thematic analysis identified three key themes relating to medication management (Box 1).
| Box 1. Key themes identified through analysis of participant interview data relating to general practice pharmacist influence on medication management of patients at risk of medication-related harm |
- Medication safety
- Prescribing continuum
- Deprescribing principles
- Shared decision making
- Challenging the status quo
- Imprimatur of prescribers
- Collegiality and teamwork
|
Medication safety
Responses from GPs, patients and carer participants contributed to the theme of enhanced medication and patient safety, consequent to GPP involvement. Improved accuracy of patients’ regimens due to medicines reconciliation was explicitly noted. GPs welcomed the objective scrutiny, recalling how discrepancies were in some cases found despite their own meticulous practice. Patients appreciated opportunities to ensure an accurate medication record and recognised potential risks from inaccuracies:
… if I’d been carted off to hospital … they’d be thinking I’ve been taking two. [Pt02]
I just felt it gave us a clean slate. [Pt09]
Mitigating possible harm for patients transitioning from hospital, specialist or other GP care was commended by GPs:
We found that the pharmacist’s interaction … showed up errors that we were making … It showed up errors that the patients were making. Then, it showed up miscommunication between the specialists, the pharmacists, and the GPs, and the patients as well. So, it made everything much more accurate and clearer in the patients’ notes. [GP09]
Frequent references were made to GPP input into targeted opioid strategies, responding to an identified practice-wide need:
I said … ‘You know what I really, really want to address is the opioid problem’. [GP05]
This initiative was particularly noted by one perceptive carer:
I got the feeling that … maybe [the practice] had been prescribing too many opioids to some of their older patients … In a practice like that, where you’ve got so many local community members, particularly an ageing population in that area … it wouldn’t hurt to have that check and balance review process. [Carer02]
Participants noted that the GPP pilot engendered additional layers of safety:
[The pilot] has really honed our approach to initial prescribing of benzos [benzodiazepines] and opioids; so that’s far more structured … Previously, certainly for myself … it was a much more limited thought process that went into prescribing them … [GP07]
Prescribing continuum
Feedback from GPs, practice personnel, patients and carers identified four domains within the prescribing continuum that had been triggered by the GPP: deprescribing, shared decision making, challenging the status quo, and an overarching requirement for prescribers’ imprimatur.
Deprescribing principles
Descriptions of GPP actions by participants included terms consistent with deprescribing fundamentals, commencing with medicine reconciliation, as lists were corrected and consolidated, redundant medicines removed and medication discrepancies resolved. Medicine reconciliation was viewed as a trigger for deprescribing, as were GPP interactions with patients transitioning through care. GPs appreciated the pharmacist initiating discussions to reduce medicines:
Sometimes you feel really nervous about bringing it up with the patient if they’ve been on it a very long time. [GP01]
With improved accuracy of medicine regimens, documenting the original purpose and prescriber was perceived as valuable, especially for more complex patients. Conversations were then noted to have segued towards medicine reduction or rationalisation:
It was really worthwhile to help [the patient] just review and remind her of what these medications were doing, or if they’re not doing anything, whether we can get rid of them or not. [Carer02]
GPs referred to opportunistic deprescribing arising from GPP interactions, as well as the specific contribution to opioid rationalisation:
We [the pharmacist and I] weaned down a lot of [the patient’s] medications and now he’s actually not on opioids at all anymore. He’s only on maybe six or eight medications and he’s doing amazingly, and his quality of life is so much better. [GP08]
Accounts were given of planning for monitoring and reassessment, subsequent to GPP deprescribing suggestions:
It was about not cutting them all out at once but reducing each of the tablets one at a time so that we could figure out whether they were actually doing anything for her or not, which made a lot of sense to me. It made a lot of sense to [the patient] as well. [Carer02]
Overall reflection from GPs and practice personnel was that substantial changes had been made, with deprescribing incorporated into the prescribing continuum:
[The pharmacist was] educating doctors … getting them to really be focusing around, not just ongoing prescribing of the medication they’ve been on for many years but really addressing the indication; why they’re on the drugs, and always actively thinking every time they come in for a script, ‘Is this working? Can we reduce this? Can we reduce the risk from these medications?’ [GP08]
Shared decision making
Feedback from many participants contributed to the concept of shared decision making, recalling how the GPP explored patients’ preferences and understanding to prompt a conscious review of the value of their current medicines. Patients welcomed their goals being considered: from the specifics of having fewer tablets, desires to find out which could be removed, to more general reflections on how the GPP interaction changed their perspectives and focus about their medicines:
That day with the pharmacist brought all my normal health habits right into sharp focus. [Pt09]
GPs and practice nurses observed how the GPP interacted with patients and investigated their goals, unconstrained by time. The value of another professional engaging with patients was recalled:
Having a different person involved, not just the doctor/patient, was a benefit to the patient. [GP10]
The pharmacist … can sit and be with the patients and … can facilitate between the GP and the patient and also work alongside our nurses and receptionists … really communicate with the GPs; so there was no barrier between staff … you could have that team approach … how patients perceive her … they respect her. [PN01]
Most accounts of outcomes from shared decision making related to opioids, particularly regarding ‘agreements’ and initiating weaning:
It’s much better when someone says, ‘Oh I didn’t realise that it could do all those things. Let’s work out how we can stop them’. [GP02]
‘I had patients who were on opioids long term … when [the pharmacist] was interacting with them, they were much more amenable to the idea of trying reductions. I had patients who were shuffling along, and really unwell and confused and when we reduced their opioid use, they improved dramatically. [GP09]
Challenging the status quo
Detailed examples and general reflections offered by all participant types developed the impression that the status quo had been challenged and older, routine clinical practices were questioned. The GPP consultations were viewed as impetus for change by GPs:
I think doctors and pharmacists live in a fairyland … We all think that patients do what we tell them, and we write the script and it’s all magic, it just happens ... I think [the pilot] just highlighted how naive we all are when it comes to patients taking their medications appropriately. [GP06]
Additionally, anecdotal observations raised the issue of questioning of a non-GP specialist:
I had one patient say … that their [non-GP] specialist … didn’t seem to like the feedback … Feedback from the pharmacist wasn’t welcome. [PN01]
Patients and carers concurred that the usual approach to medication management was challenged, which then allowed for shared decision making. They reflected on their prior trust in ‘accepting advice without questioning’ when ‘doctors tend to issue repeats without asking too many questions’ (Pt06), and noted ‘some doctors dish out pills here, there and everywhere’ (Pt08). Their feedback implied this ‘habitual’ approach and ‘routineness’ had been challenged, as the medication review by the GPP presented a ‘clear picture’ by questioning medications they had long taken with ‘little thought’, and put them ‘centre stage’. A carer described the GPP interaction as a ‘wake-up call’:
Up until that point … I just assumed the status quo … I need to actually be paying a bit more attention to this … There’s a pattern that patients fall into when you go to see your regular doctor ... there’s a kind of routineness of it … things might slip through the cracks … It made us all pay attention a little bit more. [Carer02]
Imprimatur of prescribers
Patients and carers were reassured when their GPs echoed proposals, strengthening decision making. Nevertheless, experiences of non-GP specialists’ feedback varied. There were instances of non-GP specialists’ support for GPP recommendations:
I gave up one [medication] … I was taking for many years … and the endocrinologist agreed. [Pt01]
However, discord with the updated practice philosophy was hinted at:
We had some difficulty in implementing [opioid changes] for people attending some of the local pain specialists. [GP02]
Collegiality and teamwork
Patients and carers observed teamwork and collaboration as medication regimens were systematically scrutinised or rationalised on GPP instigation, identifying this as an ‘additional service’ being offered by the practice. GPs and practice personnel referred to a team approach, with the GPP working alongside them and with patients to resolve misunderstandings and respond to their needs. A collegial nature was alluded to on several occasions as the GPP provided corroboration of actions:
It felt … less isolated … not just you, sitting in your room scribbling away. [GP05]
This sentiment echoes a patient observation:
I think GPs and medical practitioners in general need … every help they can get … Having pharmacists … more of a collegiate mentality … that’s highly desirable. [Pt10]
The single suggestion of any possible professional role infringement was by one patient; that, although the expertise of the GPP was appreciated:
‘I wouldn’t necessarily want a pharmacist to take over [my doctor’s] role’ [Pt02].
Discussion
This study presents perspectives of GPs, general practice personnel and patients and carers, on the influence of a GPP model of care in managing patients at risk of harm due to their medicines and clinical circumstances. The key findings suggested by participant feedback are of enhanced medication safety achieved through collaboration of the co-located GPP, strengthened by improved accuracy and tackling of polypharmacy, assisted by shared decision making. The areas targeted were consistent with the priority areas recommended by the Australian Commission on Safety and Quality in Health Care: to monitor and respond to inappropriate polypharmacy, reduce harm from high-risk medicines, including opioids, anticoagulants and antipsychotics, and improve medication safety at transitions of care,19 in its response to the World Health Organization 2017 global patient safety challenge to reduce medication-related harm.9
The intertwined themes the study identified align with the conceptual model of interprofessional shared decision making as developed by Légaré et al.20 In our study, shared decision making was recalled as having occurred throughout the prescribing continuum, including for deprescribing, commencing with the ‘clean slate’ enabled by medicines reconciliation. Rose et al21 advanced the ideal of an accurate reconciled medicine list to inform external providers and from which deprescribing is more attainable, a view similar to that of our participants. The presence of multiple prescribers and deficient interprofessional communication have been implicated as challenges to deprescribing.22 In our study these were regarded as triggers for medicines reconciliation and, potentially, deprescribing. GPP activity was described at various steps in the shared decision-making model: as an interprofessional initiator, at times as a support in encouraging patient and carer participation, but also as a means of monitoring outcomes. This model differs from previous studies on shared decision making for deprescribing, which are commonly limited to prescribers, patients and carers.23
The study identified that, consequent to the GPP pilot, the status quo had been challenged in pursuing medication safety. This finding contrasts with some barriers identified in studies into deprescribing in primary care. A prescriber culture of ‘don’t rock the boat’ has been identified22 and, specifically for opioids and benzodiazepines, one of ‘inertia’ for the management of patients who appeared otherwise stable or who had specialist prescribers.24 Regarding relevant perspectives arising from carer and patient feedback, comparable studies to ours have investigated patient perspectives of GPPs, notably one Australian study.25 Themes identified in our study align with those from similar research. However, our participant observations of routineness being challenged appear previously undescribed in these studies – perhaps because of the paucity of research into deprescribing through interprofessional shared decision making.
Our findings support the notion of an embedded GPP as a colleague in the interdisciplinary team. Pharmacist co-location has been viewed as crucial for implementing interprofessional GPP models in general practice.25 A similar qualitative finding from 10 Dutch general practices was that the GPP role represented an important transition from medication-focused care to person-centred care, enabled by co-location, which improved collaboration and aligned ‘professional identities’.26 Of note, a significant quantitative finding of the Dutch study was the reduction in hospitalisation found for patients at risk of medication-related harm when compared to their risk under usual care.26
Conversely, participants in our study in some cases positioned the non-GP specialist as external to this diversified general practice team, which impacted the ‘environment’ of shared decision making as described in the Légaré model.20 Needing non-GP specialist endorsement is not new in general practice research, especially in relation to opioid management. In the Australian context, GP autonomy was questioned when they reported feeling ‘obliged’ to continue opioids commenced by non-GP specialists, even when the GPs believed it was unsafe.27
Although an emerging scope of practice for pharmacists in primary care, there are few published studies in which GPPs collaborate in opioid management, especially outside of the USA.28 Our findings highlighted GPP contributions to this important aspect of patient safety through shared decision making, collaboration and deprescribing. In contrast, for various reasons, shared decision making to improve opioid safety was found lacking by general practice registrars, self-reported in a recent Australian qualitative analysis.29
Strengths and limitations
Our study explored the perspectives of all GPs and most practice personnel who experienced the pilot. Additionally, investigating the viewpoints of patients and carers strengthens the validity of the findings, with little evidence of discordance. The pilot was undertaken in a single Australian general practice and experiences may differ for GPs, practice personnel, patients and carers of other primary care settings. External pressures (bushfires and a pandemic) delayed evaluation, and deviant or additional patients’ perspectives may have been gathered with earlier recruitment. The analysis was of data collected from an interview schedule and prompts that were not modified throughout the study.
Implications for general practice
International primary care medication safety programs have organisational, professional and patient components to implementation, with pharmacists integrated into complex interventions.30 Within Australian general practices, current opportunities to mitigate risk of medication-related harm include reducing complexity of regimens,31 Home Medicine Review31 and practice clinical audits.32 Although explicit tools are available to help detect and manage problematic polypharmacy, these may not be as useful in routine practice and outside of research settings.2 Our study describes the successful implementation of one GPP model of care. Embedding a GPP in the practice team, as described in this single-centre study, has the potential to complement available approaches in managing the at-risk patient. The findings of our study add to previously published Australian and international evidence in support of this evolving role.25,26,33,34
An identified barrier to more widespread uptake in Australia is the current lack of sustainable funding, discussed at length by Deeks et al in their quantitative evaluation of a GPP model.33 Research and project grants, particularly from primary health networks, and internal funding from practices with experience of the GPP model and its benefits, remain the predominant sources of funding.