Discussion
While most GPs surveyed had good knowledge, notable gaps were identified. Further efforts are needed to promote awareness of recommendations, particularly for immunocompromised individuals. The availability of Shingrix, a non-live recombinant subunit zoster vaccine, in the private market provides an alternative, especially for immuncompromised patients.
Live attenuated herpes zoster vaccine (Zostavax [CSL/Merck]) was included on the Australian National Immunisation Program (NIP) from 1 November 2016 for adults aged 70 years, with a catch-up program for adults aged 71–79 years funded until October 2021. The catch-up program has now been extended for a further two years to 31 October 2023. The safety profile of Zostavax is well documented, with serious adverse events infrequent and predominantly in individuals contraindicated to receive the vaccine.1–5 In particular, use of the vaccine is contraindicated in people who are immunocompromised, due to the risk of unchecked vaccine virus replication causing disseminated disease.6
In March 2017, following the death of an immunocompromised person who received Zostavax despite a contraindication, the Therapeutic Goods Administration (TGA) issued a safety alert emphasising that the vaccine should not be used in patients with compromised immune function.7–9 The Australian immunisation handbook guidance on use in immunosuppressed individuals was expanded, with inclusion of a table providing recommendations in the context of use of a range of immunosuppressive agents. A pre-vaccination screening tool for immunisation providers, especially focused on general practitioners (GPs), was also developed.6,10
The TGA issued further safety alerts in July 2020 following a second death due to complications arising from vaccine virus infection. This individual was on low-dose immunosuppressants and received Zostavax in line with existing recommendations.11 In December 2020 a third Zostavax-related death12 occurred, in a significantly immunocompromised person.
Our previous survey of Australian GPs in late 2017 identified a range of knowledge gaps, including 5% incorrectly reporting that immunocompromise is not a contraindication to vaccination with Zostavax.13 Following the TGA safety alert in July 2020, we aimed to survey GPs again to assess any changes to their knowledge and behaviour.
Methods
We conducted a national online survey using SurveyMonkey between 20 and 27 October 2020 that included questions on GP demographics, and knowledge and clinical practice related to zoster vaccination. This was an anonymous voluntary survey. No identifiable information was collected in this survey. Respondents self-identified themselves as a GP (including GP registrar or GP locum). The format and content of the survey was developed in consultation with experts in the field. Five clinical scenarios were added to this survey to further assess knowledge on contraindications to Zostavax. The questionnaire was brief and completed in approximately 10 minutes. The survey was distributed by Healthed (a private national health education provider) to attendees after a routine immunisation educational webcast (that did not include any information on zoster vaccination) on 20 October 2020. The online survey closed on 27 October 2020.
Descriptive analysis was undertaken using SPSS version 25 and Microsoft Excel.
Ethics approval
Ethics approval was provided by the Sydney Children’s Hospitals Network Human Research Ethics Committee (HREC/17/SCHN/376).
Results
Of 605 GPs attending the webcast, 502 responded to the survey (response rate 83%). Of these, 52.7% were aged 55–74 years and 62% were female. Most were from either New South Wales (38.4%) or Victoria (36.5%; Table 1). Most respondents had administered zoster vaccine in the previous 12 months (84.9%; 426/502), but almost half (43%; 190/446) had never reported zoster vaccination data to the Australian Immunisation Register (AIR).
| Table 1. Demographic characteristics of general practitioners (n = 502) |
| Characteristic |
n (%) |
| Age group (years) |
|
| 25–34 |
7 (1.4) |
| 35–44 |
55 (11.0) |
| 45–54 |
120 (23.9) |
| 55–64 |
163 (32.5) |
| 65–74 |
124 (24.7) |
| 75–84 |
28 (5.6) |
| ≥85 |
5 (1.0) |
| Sex |
|
| Male |
191 (38.0) |
| Female |
311 (62.0) |
| State/territory |
|
| Australian Capital Territory |
7 (1.4) |
| New South Wales |
193 (38.4) |
| Northern Territory |
2 (0.4) |
| Queensland |
54 (10.8) |
| South Australia |
27 (5.4) |
| Tasmania |
5 (1.0) |
| Victoria |
183 (36.5) |
| Western Australia |
31 (6.2) |
| Location of practice |
| Capital city |
299 (59.6) |
| Country town (population <5000) |
36 (7.2) |
| Small city (population 5000–14,999) |
48 (9.6) |
Large city (population
15,000–50,000) |
55 (11.0) |
| Major city (population >50,000) |
53 (10.6) |
| Remote area |
10 (2.0) |
| Very remote area |
1 (0.2) |
In relation to knowledge of recommendations on zoster vaccination, most respondents were aware that zoster vaccine is NIP funded and recommended for adults aged 70–79 years (88.5%; 393/444) and that it is recommended but not funded for those aged 60–69 years (87.9%; 392/446; Figure 1). Approximately two-thirds correctly responded that the vaccine is not routinely recommended for adults aged 50–59 years (65.9%; 290/440) and not recommended for people aged <50 years (62.2%; 272/437), and half that it is recommended but not funded for those aged ≥80 years (54.3%; 240/442). Approximately two-thirds of respondents were aware that zoster vaccine can be co-administered with influenza or pneumococcal vaccine (70.7%; 311/440), with 13.4% (59/440) reporting that it cannot be co-administered and 15.9% (70/440) that they did not know (Figure 1).
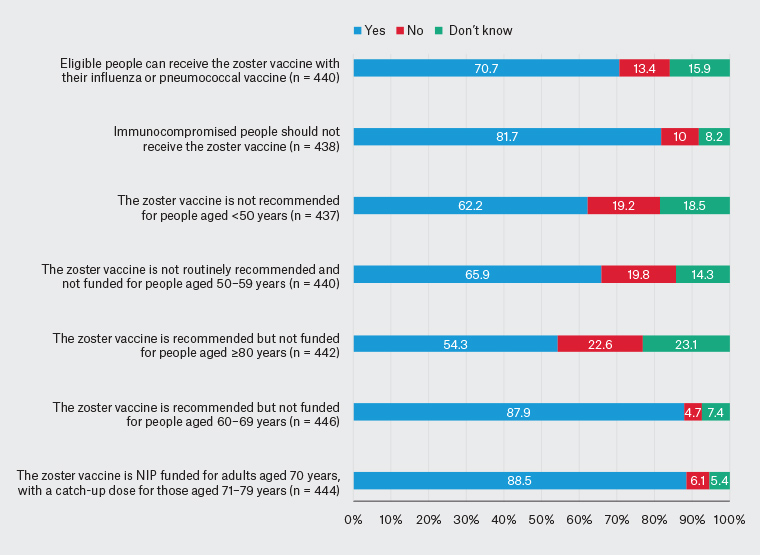
Figure 1. General practitioners’ knowledge regarding zoster vaccination. Click here to enlarge.
NIP, National Immunisation Program
Of the 438 respondents who answered the question on immunocompromise, most (81.8%; 358) correctly responded that this is a contraindication to zoster vaccination; however, 10.0% (44) stated that it is not a contraindication and 8.2% (36) did not know (Figure 1). Only 40.6% (185/456) reported being aware of safety alerts.
Across the five clinical scenarios based on guidance in the Australian immunisation handbook that were provided to more comprehensively assess knowledge on contraindications to zoster vaccination, the proportion of incorrect responses ranged from 6.7% to 56.9% and ‘don’t know’ responses from 11.3% to 19.4% (Table 2):
- Scenario 1 (male aged 70 years with rheumatoid arthritis on methotrexate 5 mg weekly): 56.9% (248/436) of GPs incorrectly responded that zoster vaccine is contraindicated and 17.9% did not know whether it is contraindicated or not.
- Scenario 2 (female aged 65 years with chronic lymphocytic leukaemia, chronic renal failure and diabetes): 14.4% (63/436) of GPs incorrectly responded that zoster vaccine is not contraindicated and 17.7% did not know whether it is contraindicated or not.
- Scenario 3 (male aged 80 years with ischaemic heart disease, atrial fibrillation and diabetes): 6.9% (30/434) of GPs incorrectly responded that zoster vaccine is contraindicated and 11.3% did not know whether it is contraindicated or not.
- Scenario 4 (male aged 45 years with Crohn’s disease on infliximab and 15 mg prednisolone daily): 6.7% (29/436) of GPs incorrectly responded that zoster vaccine is not contraindicated and 15.8% did not know whether it is contraindicated or not.
- Scenario 5 (female aged 71 years with asthma on weaning steroid dose, currently 25 mg daily): 34.9% (151/433) of GPs incorrectly responded that zoster vaccine is not contraindicated and 19.4% did not know whether it is contraindicated or not.
| Table 2. General practitioners’ knowledge assessed using clinical scenarios on contraindications to zoster vaccination as described in the Australian immunisation handbook (with correct answer shaded in grey) |
| Clinical scenarios |
Contraindicated |
Able to be given (not contraindicated) |
Don’t know |
| Male aged 70 years with rheumatoid arthritis on methotrexate 5 mg weekly (n = 436) |
248 (56.9) |
110 (25.2) |
78 (17.9) |
| Female aged 65 years with chronic lymphocytic leukaemia, chronic renal failure and diabetes (n = 436) |
296 (67.9) |
63 (14.4) |
77 (17.7) |
| Male aged 80 years with ischaemic heart disease, atrial fibrillation and diabetes (n = 434) |
30 (6.9) |
355 (81.8) |
49 (11.3) |
| Male aged 45 years with Crohn’s disease on infliximab and 15 mg prednisolone daily (n = 436) |
338 (77.5) |
29 (6.7) |
69 (15.8) |
| Female aged 71 years with asthma on weaning steroid dose, currently 25 mg daily (n = 433) |
198 (45.7) |
151 (34.9) |
84 (19.4) |
The majority of respondents had not reported any adverse reaction to Zostavax to the TGA (83.1%, 373/449) or to their state/territory public health authority (84.1%, 375/446) over the previous 12 months, noting that the majority had not seen any adverse events (Figure 2). Only 38.2% (174/455) had seen a minor adverse reaction and 12.3% (56/455) had seen a severe adverse event following Zostavax vaccination.
Further analysis limited to those GPs who reported administering Zostavax over the previous 12 months showed similar findings to those overall (data not presented).
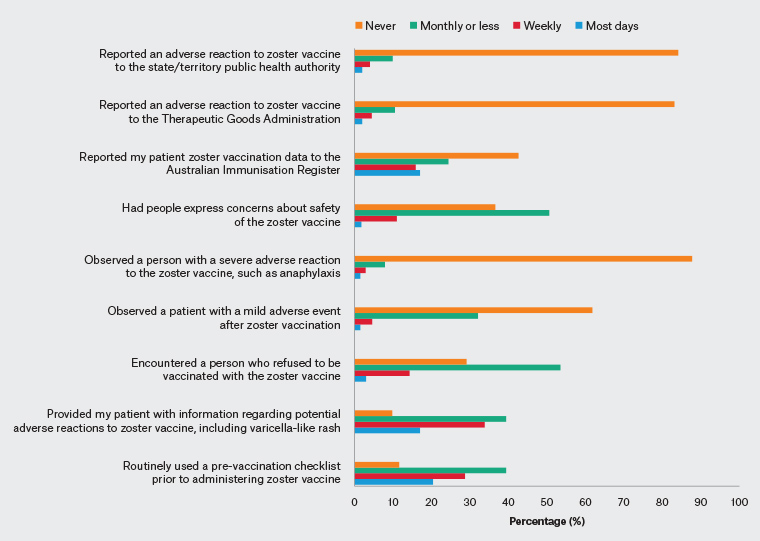
Figure 2. General practitioners’ immunisation-related behaviour in the past 12 months. Click here to enlarge.
Discussion
We found that most GPs surveyed had good knowledge of age-based recommendations in relation to zoster vaccination. However, a substantial proportion (one in five) either responded that Zostavax can be given to immunocompromised people or were unsure, a similar figure to that from our previous 2017 survey.13 Notably, two-fifths of respondents were unaware of the recent safety alerts triggered by Zostavax-related deaths in immunocompromised people.
For the five clinical scenarios used to more comprehensively assess knowledge of Zostavax contraindications, the proportion of correct responses ranged 25–82%. Similar issues with knowledge gaps about the contraindications to live attenuated zoster vaccine have been reported in other countries including Italy14 and the USA.15,16 Zostavax-related deaths in immunocompromised individuals incorrectly administered the vaccine have also been reported in Canada17 and the UK.18
Assessment of level of immunocompromise can be complex and challenging. In addition, one of the Australian Zostavax-related deaths was in a person reported to be relatively mildly immunocompromised (was taking hydroxychloroquine and a low dose of prednisolone to treat arthritis)11 and not contraindicated for vaccination under existing guidelines. Our findings suggest that additional efforts are required to improve point-of-care precision in assessment of eligibility, contraindications, and secondary validation of these. This should include promotion of greater awareness and understanding of Australian immunisation handbook zoster vaccination recommendations6 and the pre-vaccination screening tool,10 and identification of further measures and resources to support clinicians in their clinical assessment and decision making, including when to seek specialist advice. GPs should clearly explain benefits and risks of zoster vaccination to ensure informed consent. Patients should be advised of the rare possibility of vaccine virus-related disease and the need for prompt medical assessment if they develop a varicella-like rash after Zostavax,19 with information provided being appropriate to their culture, language and literacy.
Some of the issues relating to use of Zostavax may be addressed through use of Shingrix, a new non-live adjuvanted sub-unit herpes zoster vaccine. Shingrix was registered in Australia in 2017 but has not been available due to global shortages.20 However, from mid-2021 it is available on the private market for use in adults aged ≥50 years, although supply is expected to be limited. The Australian Technical Advisory Group on Immunisation (ATAGI) has advised that Shingrix is preferred over Zostavax for the prevention of herpes zoster and associated complications in people aged ≥50 years, although Zostavax remains an effective alternative vaccine for immunocompetent adults.21 In mildly immunocompromised adults, ATAGI advises that Zostavax may be administered when Shingrix is not accessible after careful assessment of the degree of immunocompromise using the Zostavax pre-vaccination screening tool.21 While the availability of Shingrix in the private market provides an alternative for those with immunocompromise, this creates equity issues due to its considerable out-of-pocket costs compared to Zostavax, which continues to be NIP funded for people aged 70 years.
Almost half of our survey respondents had not reported zoster vaccination data to the AIR in the previous 12 months, consistent with a previous report identifying substantial under-reporting.22 Data completeness should improve with the introduction of mandatory reporting of vaccinations to the AIR from 1 July 2021 for all vaccine providers and all NIP vaccines.23 Similarly, online reporting of adverse events following immunisation should be encouraged to detect and investigate safety signals.
Improvement of processes would be facilitated by clinical software prompts/flags for immunodeficiency once intention to immunise is recorded; use of immunisation/practice champions to improve adherence to protocols from pre-immunisation through to post-immunisation; and formal accreditation processes to ensure integrity. Similar to COVID-19 vaccination, mandatory training and certification of immunisers could be undertaken for zoster vaccine (and potentially all NIP vaccines), with regular mandated training updates. Further practice-based research to inform the optimisation and dissemination of best practice protocols would also likely be of benefit.
While our survey had a good response rate (83%), the participating GPs may not be representative of all Australian GPs. Respondents were older than Australian GPs overall (64% versus 37% aged ≥55 years) and with a higher proportion female (62% versus 47%).24 Also, GPs who attend educational webinars could differ in their knowledge compared to GPs overall. However, our survey demonstrates that the results are reliable and consistent with our previous survey.13
In conclusion, we encourage GPs to make use of key zoster vaccination resources including the Australian immunisation handbook, updated ATAGI clinical advice and pre-vaccination screening tool, and to seek specialist advice where uncertain about level of immunocompromise and appropriateness of Zostavax. Additional or enhanced resources and support may be helpful given the complexity of assessment, and development could be informed by in-depth qualitative research with GPs. A comprehensive analysis of rates and outcomes of Zostavax administration errors, using TGA and AIR data, is also warranted. The availability of Shingrix in the private market provides another vaccination option, but also creates issues of equity of access until, and if, it is included on the NIP.