Case
A man aged 78 years presented with a two-week history of erythematous urticarial patches and plaques that had started on the feet and spread to the legs, arms and hands. Many of these lesions had evolved into both intact and ruptured bullae (Figure 1). He reported feeling well otherwise and denied recent fevers. His medical history included Parkinson’s disease, gastritis and type 2 diabetes. A review of his medications revealed that he was taking levodopa, carbidopa, temazepam, pantoprazole and metformin long term. He was also commenced on sitagliptin eight months prior to presentation and had self-medicated with ibuprofen over the past two weeks for knee pain.
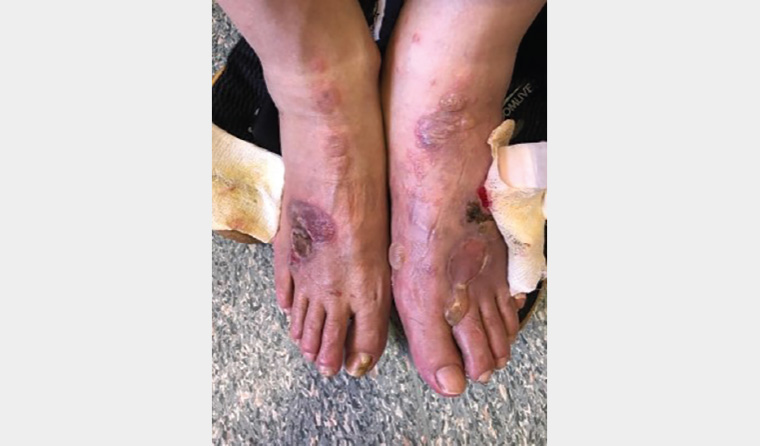
Figure 1. Intact and ruptured bullae on the feet
Question 1
What conditions can present as an eruption of bilateral tense bullae?
Question 2
What is the most likely cause in this case?
Question 3
What investigations would you order?
Question 4
What red flag signs or symptoms might you be concerned about?
Answer 1
Identifying the level of skin at which blisters occur is of primary importance in formulating a list of differential diagnoses. The presence of tense and intact bullae indicates a separation at the dermo-epidermal junction or subepidermal levels, whereas fragile blisters that rupture with gentle rubbing (Nikolsky’s sign) indicate an intraepidermal separation.
Differential diagnoses of widespread bullae on the limbs include:
- bullous pemphigoid (BP): The most common autoimmune blistering condition. It is characterised by autoantibodies specific for skin basement membrane, causing subepidermal blisters
- drug eruption: This includes bullous lichenoid eruptions, fixed drug eruptions, as well as photosensitising drug reactions, and typically exhibits tense bullae. Fixed drug eruption is less likely in this context, given the widespread distribution
- bullous insect bite: Acute onset of itchy papules in clusters, which evolve into blisters and might be tense and persist for several days. Could show linear distribution and more commonly affect exposed areas. A history of exposure to potential offenders is an important clue
- epidermolysis bullosa acquisita: A rare autoimmune condition that induces tense bullae at sites of minor trauma, commonly hands, feet, knees, elbows and buttocks, with occasional mucosal involvement. Can occur at any age, but peak incidences have been reported to be in the second and seventh decades of life1
- mucous membrane (cicatricial) pemphigoid: A rare autoimmune blistering condition that most commonly causes subepidermal blistering on mucous membranes, but can also affect skin. Mean onset is in the seventh decade of life2
- bullous lupus erythematosus: A rare manifestation of systemic lupus erythematosus characterised by subepidermal bullae and autoantibodies to components of type VII collagen
- drug-induced pemphigus: An autoimmune reaction to desmoglein proteins within the epidermis, causing fragile intra-epidermal blisters. Given this patient had tense blisters, this diagnosis is unlikely.
Answer 2
The most likely diagnosis in this patient is BP. BP is the most common autoimmune bullous disease and is most prevalent in those aged over 60 years (median age of onset 65 years) and who have a neurological disease, such as stroke, dementia or Parkinson’s disease.3 Given the patient’s medication history, drug-induced BP should be considered.
The most likely causative drug in this case would be the patient’s dipeptidyl-peptidase 4 (DPP4) inhibitor, given the time of onset from commencement. Median onset time from DPP4 inhibitor commencement has been reported to be 11 months; however, this can range from 10 days to three years.4 This wide range is likely due to the autoimmune nature of the disease and time required for sufficient production of autoantibodies, which could vary significantly in individual cases. Clinically, lesions of DPP4 inhibitor–induced BP are also more widespread than traditionally described BP, as in this case.4
Other medications commonly associated with BP include diuretics, such as frusemide and spironolactone, antibiotics, non-steroidal anti-inflammatories, and immunomodulators, such as antitumour necrosis factor α agents and programmed cell death protein 1 (PD-1) inhibitors.5 Although the patient had also used ibuprofen, this is unlikely to be the cause given the recency.
Answer 3
Skin biopsy is required for the diagnosis of BP. A punch biopsy through the edge of an active erythematous blister (ideally within 24 hours of appearance of the lesion), including a small portion of adjacent normal skin, should be taken for haematoxylin and eosin staining, and a second from normal-appearing skin within 1–2 cm of the lesion for direct immunofluorescence (DIF).6 An alternative would be a large punch biopsy taking a whole, small intact blister and some marginal skin.
Specific blood tests for BP180 and 230 antibodies should be ordered to confirm the cause. Blood tests for full blood count; electrolytes, urea and creatinine; and liver function tests should be taken for baseline renal and hepatic function in case there is a need to commence systemic medications. An immunosuppression screen, including hepatitis B, hepatitis C, human immunodeficiency virus and QuantiFERON Gold, is also advised, as patients might require prompt immunosuppressive medications.
Answer 4
Infection and sepsis are major sequelae of concern in BP, given the risk of skin barrier breakdown.7 Prompt diagnosis and treatment to ensure fast resolution of old lesions and suppression of new lesions are key to minimising complications. Monitoring for signs of local infection, as well as systemic signs such as fever, is important.
A small percentage of BP has been reported to be associated with internal malignancy, although the evidence to date is not conclusive.8 Clinicians should keep this in mind in patients with biopsy-confirmed BP that is not responding to escalations in treatment as a rarer potential driver of the disease.
Case continued
Skin biopsies were taken from the patient and showed a subepidermal bulla and moderate perivascular inflammatory infiltrate comprising lymphocytes, plasma cells and eosinophils. In addition, there was also a mild neutrophilic and eosinophilic infiltrate within the bulla. DIF showed linear complement 3 deposition along the dermo-epidermal junction and no evidence of immunoglobulin A or G (Figure 2). The histology, DIF and indirect immunofluorescence results confirmed the clinical diagnosis of BP. The patient’s blood results were positive for BP180 (index 8.21) and 230 (index 3.16) antibodies, performed with enzyme-linked immunosorbent assay. Blood counts and renal and liver function tests were normal. The patient was afebrile, and none of the lesions exhibited signs of infection.
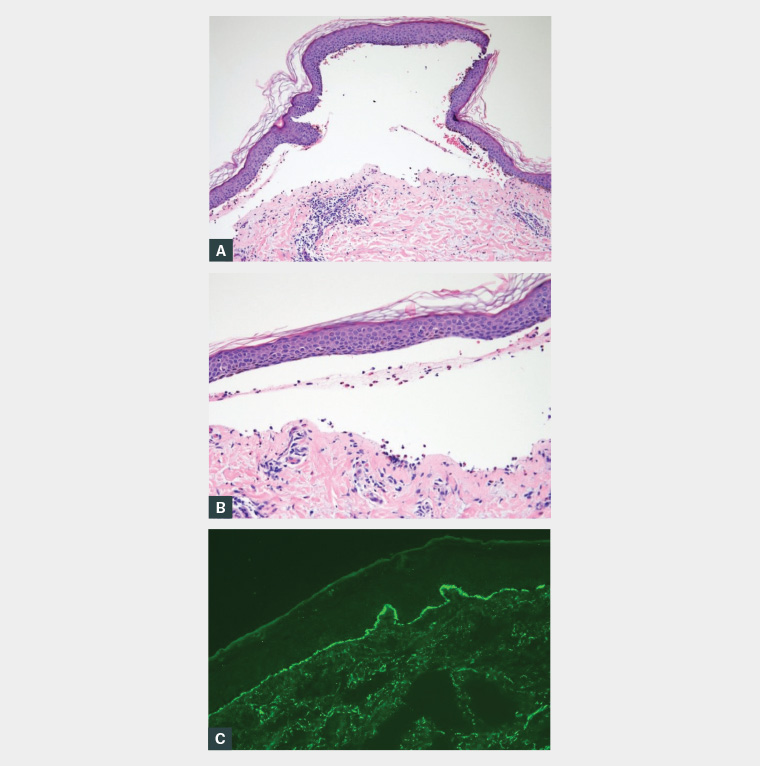
Figure 2. Subepidermal blister with perivascular and dermal neutrophilic, lymphocytic and eosinophilic infiltrate
A. 10× magnification; B. 20× magnification; C. Linear complement 3 deposition at the dermo-epidermal junction on direct immunofluorescence
Question 5
What treatment options are available for BP?
Answer 5
Potent topical corticosteroids, such as betamethasone dipropionate, ideally with wet wrap occlusion, are considered a first-line treatment; however, their efficacy is reliant on consistent application and could present difficulties in those with extensive disease.9,10
Most patients with moderate-to-severe disease will require at least some dose of oral corticosteroids. The aim of oral corticosteroids should be to establish prompt remission and then wean to the lowest possible dose to maintain control with the aid of steroid-sparing adjuvants to reduce steroid-associated side effects. Starting doses of corticosteroids should be limited to under 0.5 mg/kg/day equivalent of prednisone. and ideally to lower doses of between 0.2 and 0.4 mg/kg/day. Studies have shown a starting dose of 0.5 mg/kg/day to be as effective as doses up to 1 mg/kg/day in achieving control and remission.9 Oral corticosteroids should then be tapered slowly to achieve a minimum effective dose for preventing new lesion formation. Tetracyclines and macrolides have been used as third-line options due to their anti-inflammatory activity, and have shown efficacy as both monotherapy and adjuvant therapy.9,10 A combination of oral nicotinamide and tetracyclines has also demonstrated equivocal effectiveness to oral prednisone in some patients.9
Other immunomodulatory adjuvants include dapsone, mycophenolate mofetil, cyclosporine, azathioprine and methotrexate. The use of these agents should be guided by a dermatologist. There is evidence for the use of intravenous immunoglobulin and biological agents, including rituximab and omalizumab, in patients with recalcitrant disease or significant side effects to other therapies.9
Case continued
The patient was commenced on oral prednisone 25 mg daily (0.5 mg/kg/day; patient weight 50 kg), doxycycline 100 mg daily and twice a day wet dressings with topical betamethasone dipropionate. While on high-dose prednisone, the patient was also advised to take a proton pump inhibitor to protect against steroid-induced gastritis, as well as vitamin D and calcium supplementation to reduce bone density loss. The patient was advised to cease sitagliptin and switch to an alternative non-DPP4 inhibitor anti-hyperglycaemic if required.
Question 6
What are important features to monitor long term?
Answer 6
BP is a chronic condition that can last for many years, during which time patients could have multiple episodes of relapse that might require treatment with systemic medications, such as immunosuppressants. It is therefore important to regularly monitor patients for disease severity and the following parameters:11
- body sites
- type of lesions: Longstanding or transient
- number of lesions
- severity of pain or itch.
For patients on systemic medications, it is important to monitor:
- blood counts and renal and liver functions
- blood pressure
- body weight
- side effects of systemic corticosteroids, including gastritis, hyperglycaemia and osteoporosis.
Case continued
The patient was followed up two weeks after commencing treatment and showed a good response. Prednisone was subsequently weaned to an ongoing dose of 5 mg daily, doxycycline 100 mg daily continued and betamethasone dipropionate ceased. Ongoing follow-up every three months was scheduled, and the patient was advised that if new lesions appear, he should recommence topical betamethasone dipropionate immediately and present for early review.
Key points
- BP is a diagnosis that is important to consider in elderly patients with recent‑onset pruritic erythematous or bullous lesions, especially in the context of associated medications, such as DPP4 inhibitors.
- Prompt treatment with immunosuppressive medications is key to reduce sequelae, and most patients will require oral corticosteroids. However, adjuvants should be used to minimise the required corticosteroid dose.
- Regular monitoring for disease activity and steroid-related side effects is important due to the chronicity of the condition and potential need for lengthy oral corticosteroid therapy.