Background
Renin–angiotensin system inhibitor (RASI) therapy is the cornerstone of chronic kidney disease (CKD) management and slowing of disease progression. However, debate remains about the use of RASI therapy in advanced CKD. Declining RASI therapy use in CKD may reflect prescribers’ lack of confidence given the absence of clear guidelines.
Objective
This article reviews evidence for RASI therapy in patients with advanced CKD, with the aim of enhancing general practitioners’ (GPs’) awareness to the cardiovascular and renoprotective benefits that extend into this population.
Discussion
There is a myriad of data supporting the use of RASI therapy in patients with CKD. However, the lack of data in advanced CKD remains a critical gap that has the potential to influence progression of disease, time to renal replacement therapy and cardiovascular outcomes. Current practice guidelines support continuation of RASI therapy in the absence of contraindications because of its mortality benefit and potential to preserve renal function.
Chronic kidney disease (CKD) is a common condition affecting approximately 1.7 million Australians. It is defined by a combination of renal function and urine protein excretion, which are used to stage disease and guide therapies (Figure 1).1 Patients with CKD are at increased risk for all-cause mortality, which, as they progress to end-stage kidney disease (ESKD), compounds the importance of optimising medical intervention. Cardiovascular risk management is of particular importance as patients with stage G3b or macroalbuminuria are classified into the highest cardiovascular disease risk group (>15% probability in five years). This manifests as patients with CKD having up to a 20 times greater risk of death from cardiovascular events before even progressing to dialysis, with a two- to threefold greater risk of cardiac death than those without CKD.2,3 No current therapy has been shown to reverse CKD; however, it is well established that renin–angiotensin system inhibition is the cornerstone of slowing early progression and optimising cardiovascular disease risk and mortality.4–6 The Kidney Disease: Improving Global Outcomes (KDIGO) 2021 CKD guidelines collate international working groups and up-to-date evidence. Box 1 summarises these recommendations and practical points for renin–angiotensin system inhibitor (RASI) use and initiation in CKD.
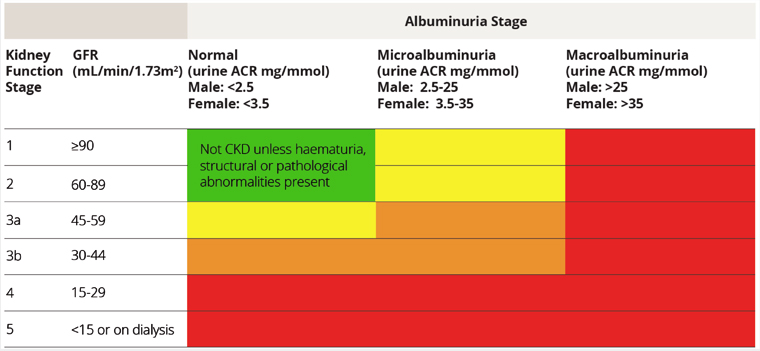
Figure 1. Grades of chronic kidney disease.
ACR, albumin-to-creatinine ratio; CKD, chronic kidney disease; GFR, glomerular filtration rate; ESKD, end‑stage kidney disease.
Reproduced with permission from Kidney Health Australia, Chronic kidney disease (CKD) management in primary care: Guidance and clinical tips to help detect, manage and refer patients in your practice with CKD, 4th edn. Melbourne, Vic: Kidney Health Australia, 2020.
| Box 1. Established recommendations for use of renin–angiotensin system inhibitor therapy in adult patients with chronic kidney disease not receiving dialysis4 |
| Recommendations |
- Adults with CKD and elevated blood pressure should be treated to target of <120 mmHg where tolerated (GRADE 2B).
- Adults with CKD G1–G4 with elevated blood pressure and severe albuminuria (A2–3) with or without diabetes (GRADE 1B).
- Adults with CKD G1–G4 with elevated blood pressure and moderate albuminuria (A2–3) with diabetes (GRADE 1B).
- Adults with CKD G1–G4 with elevated blood pressure and moderate albuminuria (A2–3) without diabetes (GRADE 2C).
- Combination RASI therapy should be avoided (GRADE 1B).
Practice points
- CKD G1–G4: Consider treatment for those with systolic blood pressure >130 mmHg in the absence of albuminuria.
- When prescribing RASI therapy, use highest tolerated dose to best replicate trial outcomes.
- Assessment of serum electrolytes, renal function and blood pressure should occur 2–4 weeks following initiation; continue medication unless serum creatinine rises > 30% within four weeks.
- Hyperkalaemia due to RASI therapy should be managed with measures aimed at reducing serum potassium rather than dose adjusting RASI therapy where possible.
- Indications to cease or reduce RASI medications are: symptomatic hypotension, uncontrolled hyperkalaemia despite medical management or reduce uraemic symptoms while treating ESKD.
|
| RASI, renin–angiotensin system inhibitors; CKD, chronic kidney disease; ESKD, end-stage kidney disease. |
Advanced CKD (G4–G5) is less clear, having been underrepresented in pivotal trials thus far.4,7 A recent report acknowledged the lack of data in this population as a critical gap in research.8 As it stands, there are only weak recommendations for RASI therapy in CKD G4 and no consensus recommendations for use in G5 because of a lack of randomised control data regarding the risk–benefit of continuation or discontinuation.4 This is reflected in practice by the robust observation of patients with CKD G3–G5 not receiving RASI therapy.7–11 The lack of data and concerns about side effects hamper prescriber confidence in using RASI therapy for advanced CKD, which may result in unnecessary morbidity and disease progression for patients.12 The aim of this article is to discuss the current evidence for RASI therapy in advanced CKD, with the goal of highlighting the proposed cardiovascular and renoprotective benefits. To this end, the aim is to provide general practitioners (GPs) with the confidence to identify patients with CKD who are not on RASI therapy and consider initiation where appropriate.
Trends of declining RASI prescription as patients progress from CKD G3 to G5 is a development worth investigating, given what is known about the cardiorenal benefits of RASI therapy in those with CKD. Renal protective benefits have been established in CKD throughout most research in the past two decades. This includes slowing disease progression to ESKD and delaying the need for renal replacement therapy (RRT). An observational study of 4803 patients with advanced CKD in the Swedish Renal Registry (SRR) compared rates of RRT and major adverse cardiovascular events (MACE) in those using RASI therapy versus calcium channel blockers.13 These authors identified that the new use of RASI therapy lowered the risk of need for RRT (adjusted hazard ratio 0.79 [95% confidence interval: 0.69, 0.89]), an effect that was seen across subgroups including diabetes, heart failure, proteinuria and diabetes. These principal findings are corroborated by recent network meta-analysis data of over 40,000 patients with G3–G5 CKD who are not receiving dialysis.14 RASI therapy preserved kidney function and reduced cardiovascular events and all-cause mortality in a population with advanced CKD.
Large observational studies have also identified increased risk of death and ESKD with discontinuation of RASI therapy, supporting the idea that RASI therapy preserves renal function as CKD progresses.9,15 A prospective cohort analysis of over 28,000 patients with advanced CKD found that RASI therapy was associated with lower risks for long-term dialysis of up to 5.5% per annum.16 The use of benazepril in advanced CKD resulted in a 40% lower incidence of ESKD, slower rate of estimated glomerular filtration rate (eGFR) decline (6.8 vs 8.8 mL/min/1.73 m2/year) and reduced proteinuria (20% vs 52%).17 Moreover, the preservation of kidney function in CKD and ESKD was also identified in the Ramipril Efficacy in Nephropathy (REIN) trial, where post-hoc analysis found that use of RASI therapy decreased incidence of ESKD in patients with CKD G4.18 Reductions in the need for RRT by up to 25% have been observed in trials such as the PRE-dialysis Patient Record-2 (PREPARE-2) study.19
Arguments against use of RASI therapy in advanced CKD highlight the risk of increased need for RRT. In 2021, Fu et al20 assessed the effect of stopping RASI therapy in 10,254 patients (eGFR <30 mL/min/1.73m2) on CKD progression and cardiovascular risk. Interestingly, the researchers’ reported cessation was associated with lower absolute risk of RRT initiation (27.9% vs 36.1%). This finding is corroborated by earlier research in which RASI therapy in patients with CKD G4–G5 had a detrimental effect of eGFR decline, with improvement of eGFR after discontinuation of RASI therapy.21 Small trials observed restoration of eGFR by 25–50% 12 months after cessation.15 On a larger scale, an observational trial of 10,254 patients over 10 years on the SRR found a reduction of 8.6 RRT events per 100 patients with cessation of RASI therapy.20 With the view of kidney function taken in isolation, these numbers may compel practitioners to keep patients off RASI therapy through the late stages of CKD.
Another argument against the use of RASI therapy in CKD is prescriber concerns about medication side effects, especially hyperkalaemia and acute kidney injury.10 Hyperkalaemia occurs as a result of decreased ion excretion with renal failure and medications such as RASI therapy. Other common side effects and considerations are outlined in Table 1. The prevalence increases as CKD progresses, and annual prevalence ranges widely between 6.35% and 20% in this cohort, although variations in medical management, polypharmacy and pseudohyperkalaemia account for some of this.22,23 To this end, patients with advanced CKD who continue RASI therapy may require increased monitoring of electrolytes and have prevention strategies in place. This may include use of potassium-binding polymers (patiromer) and established ‘sick-day’ plans for dose reduction or temporary withdrawal.1,4,24 Table 2 summarises considerations for adjustment during illness.
| Table 1. Common side effects and considerations for use of renin-angiotensin system inhibitor therapy in advanced chronic kidney disease26 |
| Side effect/complications |
Considerations |
| Hypotension |
- More common in elderly patients
- Associated with polypharmacy and comorbid disease (eg heart failure)
- Influences activities of daily living and independence, and increases falls risk
- Caution should be used with systolic blood pressure <90 mmHg
|
| Hyperkalaemia |
- Prevalence increases with loss of renal function
- Can be precipitated by common medications: potassium-sparing diuretics, NSAIDs, trimethoprim–sulfamethoxazole
- Prevention with low salt and low potassium diets, medication reconciliation (eg spironolactone, eplerenone)
- Possible role for cation exchange resin (patiromer) in chronic hyperkalaemia
- Caution should be used for those with a serum potassium level >5.5 mmol/L
|
| Acute kidney injury |
- Common with volume depletion, summer months (dehydration)
- Caution for those with renal artery stenosis
- More common in those with polypharmacy as a result of drug–drug interactions
- Recognition of acute illness and having a ‘sick-day plan’ in place to alter medications has an important role in prevention
|
| Drug–drug interactions |
- Analgesia: NSAIDs/cyclooxygenase-2 inhibitors
- Diuretics: thiazide agents, loop diuretics, potassium-sparing diuretic
- Secondary RASI agent or angiotensin receptor-neprilysin inhibitors
- Antihyperglycemic agents: sodium–glucose co-transporter-2 inhibitors
|
| Cough |
- Discontinue ACE inhibitor and change to alternative such as ARB
|
| Angioedema |
- Most commonly affects lips, tongue and upper airway
- Discontinue ACE inhibitor
|
| ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor blocker; NSAIDs, nonsteroidal anti‑inflammatory drugs; RASI, renin–angiotensin system inhibitor. |
| Table 2. Sick day identification and management of common medications in chronic kidney disease1 |
| Who is at risk |
All patients with chronic kidney disease (especially G3–G5)
Comorbid disease
- Diabetes
- Heart disease
- Lung disease
- Liver disease
- Malignancy
Elderly individuals
Female sex |
| Common triggers |
Acute illness
Dehydration or hypovolemia
- Vomiting, diarrhoea
- Poor fluid intake associated with illness, heat-related illness (summer months)
|
| Common medications that require dose reduction or cessation (guided by baseline renal function) |
Blood pressure
- Angiotensin-converting enzyme inhibitors
- Angiotensin receptor blocker
- Loop diuretic
- Thiazide diuretic
- Potassium-sparing agent
Diabetes
- Metformin
- Sulfonylurea
- Sodium–glucose co-transporter-2 inhibitors
- Sitagliptin
- Insulin
Other
- Nonsteroidal anti-inflammatory drugs
- Statins
- Trimethoprim
|
| Resources for management of chronic kidney disease and intercurrent illness |
- Drug adjustment: https://renaldrugdatabase.com
- Drug adjustment: https://amhonline.amh.net.au.acs.hcn.com.au
- Sick day patient information: https://kidney.org.au
- Sick day guidance: www.thinkkidneys.nhs.uk
- Sick day guidance: www.cariguidelines.org
|
After acknowledging the risk of medication side effects in advanced CKD, it is KDIGO’s stance that well-informed patients with advanced CKD may be more likely to value the chance of cardiorenal protection over potential reversible harms.2 This is understandable, especially when one considers that cardiovascular disease is the leading cause of death in patients with CKD, not hyperkalaemia.1 In support of this, we focus on the evidence that discontinuation of RASI therapy is associated with mortality, MACE and CKD progression.9,13 However, CKD rarely occurs in isolation; therefore, it is critical that clinicians value the ‘whole of person’ approach to medical management of CKD as outlined in Australia guidelines,1 given the commonly associated multimorbid burden (Table 3). GPs and non-GP specialists can help educate patients and families on making informed choices on the basis of available evidence.
| Table 3. Chronic kidney disease and prevalence of most common comorbid conditions*27 |
| Condition |
Prevalence |
| Hypertension |
91.6–94.5% |
| Hypercholesterolaemia |
38.8–50.5% |
| Diabetes |
21.4–45.1% |
| Coronary artery disease |
21.0–26.7% |
| Cerebrovascular disease |
5.7–11.1% |
| Congestive heart failure |
7.5–13.5% |
| *Data representative across two separate studies: The Kidney Early Evaluation Program 2010 and National Health and Nutrition Examination Survey 1999–2006. |
Conclusion
The observation of declining use of RASI therapy as CKD progresses is likely rooted in prescriber concern for side effects and influence on disease progression as a result of research gaps. While there may be an absence of randomised trials, several large trials have noted positive effects of RASI therapy extending into advanced CKD and ESKD. Data supporting the efficacy of RASI in patients with CKD reflect the renal and cardioprotective properties of treatment. Because of this, our authors support current KDIGO practice points.
4 Cessation of RASI therapy in stable patients with CKD may unnecessarily deprive many patients of the cardiovascular benefits and renoprotective properties they would otherwise receive. Practically, it is important for both physicians and patients to appreciate that most do not survive to ESRD because of cardiovascular mortality. This fact should be central to discussions with patients and GPs, juxtaposing the risks of medication side effects; well-informed patients may be more likely to proceed with RASI therapy. We recommend all clinicians involved in the care of such patients aim to identify those not on RASI therapy and review their use in the context of each individual. The same points should be considered prior to ceasing therapy. Before making a decision, the complex state of patients with CKD must be considered, including: multimorbidity, polypharmacy, functional status, blood pressure, goals of care and increased need for monitoring. Flexible and premediated strategies for preventing and managing complications such as hyperkalaemia should be in place. Moving forward, clarity on the benefit of RAS inhibition in advanced CKD will come from the results of the STOP-ACEi trial, a multicentre randomised control trial currently underway studying the discontinuation of RASI in patients with CKD G4–G5.
25
Key points
- There is a lack of randomised control data regarding the risk–benefit for continuation or discontinuation of RASI therapy in advanced CKD.
- From the available evidence, current guidelines support RASI therapy in advanced CKD given the beneficial cardiovascular and renoprotective effects.
- GPs are well placed to identify patients with CKD who are not on RASI therapy, considering the use of RASI therapy in the context of multimorbid disease and potential side effects.
- Prevention of complications such as hyperkalemia requires increased electrolyte monitoring with flexible and premediated strategies during periods of illness (ie sick-day guidance).
- Complex decisions regarding continuing RASI therapy should be made in collaboration with other care providers including nephrologists and renal support teams.