Fibromyalgia (FM) is a chronic pain disorder characterised by widespread musculoskeletal pain, fatigue, cognitive disturbance and psychiatric symptoms.1 The prevalence of FM within the general population ranges from 1.3% to 8%, and FM appears to be more common in women.3 FM is a disabling syndrome, with approximately one-third (30.2–34.8%) of patients in large North American studies having their ability to work affected to the point of requiring supplementary disability income support.4 A recent Australian survey showed a similar rate (35.1%) of financial disability support in FM patients with one-quarter (24.3%) stopping work and one-third (32.6%) reducing paid work within 5 years of symptom development.5 FM is currently diagnosed using the 2010 American College of Rheumatology (ACR) criteria, including the widespread pain index (ie the number of locations of pain) and the symptom severity scale (other FM systemic symptoms).1,2
Pathophysiology of FM
The precise pathogenesis of FM remains unclear, but three main mechanisms have been hypothesised in the literature, namely altered central nervous system (CNS) sensory synaptic transmission, autonomic system dysfunction and peripheral neurogenic inflammation (Figure 1).
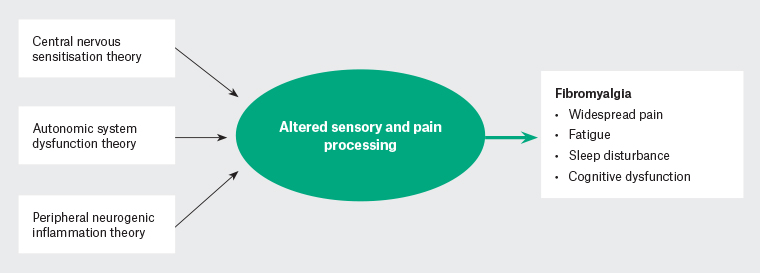
Figure 1. Hypothesis of fibromyalgia pathophysiology.
Altered CNS sensory synaptic transmission is evidenced by increased temporal summation of noxious stimulus (increased pain on repeated stimulation) and allodynia. This may be explained by deficient endogenous analgesic system function (changes in endorphins and enkephalins);3 changes in opioid receptors and pain-related neuropeptides;3,6 and changes in immune function and reported consistent increases in serum concentrations of proinflammatory cytokines, such as interleukin (IL)-1 and IL-6, leading to microglial activation, which can increase synaptic glutamate release, increasing central nociceptive neurotransmission.7,8
Increased levels of proinflammatory and pronociceptive markers are also measured in peripheral samples from FM patients, indicating that both central and peripheral pain sensitisation processes may be contributing to the FM syndrome.9
Several investigators have viewed FM as a stress-related dysautonomia with nociplastic pain features.10,11 Chronic sympathetic hyperactivity results in alterations in dorsal root ganglia and abnormal connections between the sympathetic and nociceptive system.11
Patients with FM often experience considerable disruptions to normal daily routines, including poor sleep, reduced activity, low mood and reduced social engagement.1 The role of these psychosocial factors in the perpetuation of the chronic pain cycle of FM cannot be overstated, and they should not be ignored in the pursuit of a solely biomedical understanding of the syndrome.
Pharmacological treatment
Medications commonly prescribed in FM include gabapentinoids (eg pregabalin and gabapentin), opioids, sedatives, antipsychotics, antidepressants of all classes and simple analgesics, such as paracetamol and non-steroidal anti-inflammatory drugs.1,3,12,13 These medications often have small effect sizes and the duration of benefit may only be a few months, often accompanied by drug dependence and side effects, such as weight gain, somnolence and reduced cognitive ability.1,3 Ironically, these side effects are often also seen as symptoms of FM; therefore, attempts to treat certain aspects of the syndrome may be worsening the overall condition.1,12,13
Clinical experience and case reports, as well as internet support groups, have led to a growing interest in low-dose naltrexone (LDN), an opioid receptor antagonist, as an off-label treatment for FM symptoms.14 LDN refers to naltrexone at doses of 1–5 mg, which has been reported to improve symptoms in patients with FM.15
This scoping review of the literature was conducted with a view to guiding clinical practice and informing future research. It aims to explore the existing literature concerning LDN use in patients with FM and the proposed mechanism of action.
Methods
The literature search of the PubMed, Cumulative Index to Nursing and Allied Health Literature (CINAHL) and Embase databases was conducted in May – July 2022, searching for publications associated with ‘low-dose naltrexone use in the management of fibromyalgia symptoms’. The following key words were used in the search: ‘low-dose naltrexone’, ‘fibromyalgia’, ‘fibrositis’ and ‘chronic pain’. A summary of search terms is provided in Appendix 1.
The literature search was conducted in May—July 2022 and identified 376 articles. Two reviewers (NA, ZL), independently screened the articles using the agreed selection criteria (Table 1), with any differences resolved through discussion. After screening the titles and abstracts, 57 articles were retrieved for full-text eligibility assessment. Following full-text review, 21 articles were included for thematic analysis (Appendix 2). Most articles were excluded from this review because of a limited discussion on LDN in FM in the context of larger considerations, such as the general treatment of FM or an approach to chronic pain. These articles were reviews or opinion articles and did not add appreciably to the understanding of the topic. A Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram outlining the included and excluded studies is shown in Figure 2.16,17
| Table 1. Selection criteria |
| Inclusion criteria |
- Peer-reviewed journal article published in English
- LDN intervention among patients with FM
- Proposed mechanism of action of LDN in FM
|
| Exclusion criteria |
- Review articles with limited content on either LDN or FM
- Conference papers or abstracts
- FM but limited content on LDN
- Chronic fatigue syndrome
- Commentary or expert opinion
- Naltrexone (50 mg) not LDN
- LDN for pain management but patients do not have FM
|
| FM, fibromyalgia; LDN, low-dose naltrexone. |
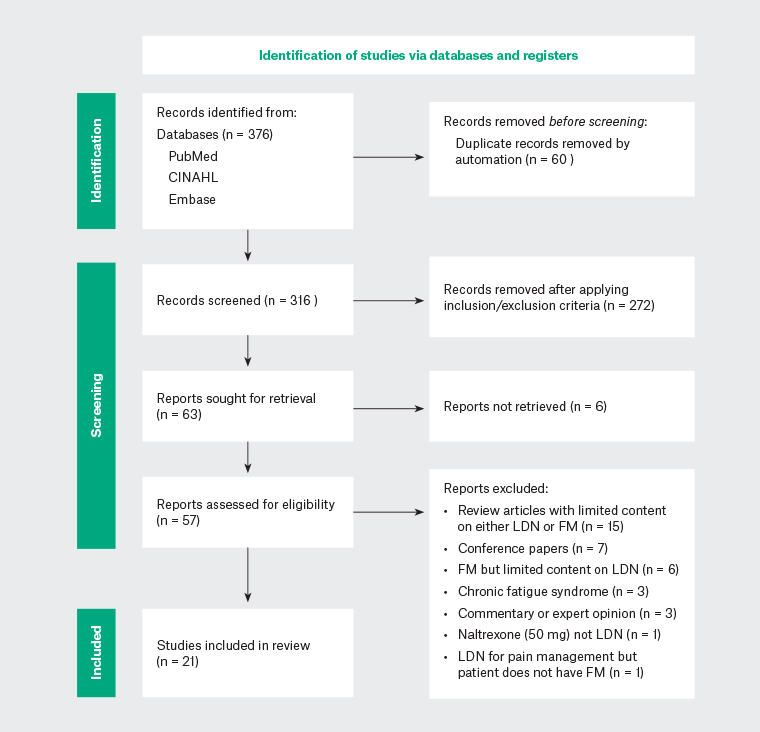
Figure 2. PRISMA flow diagram of included and excluded studies.
CINAHL, Cumulative Index to Nursing and Allied Health Literature; FM, fibromyalgia; LDN, low-dose naltrexone.
Results
After screening, 21 papers were found to meet the review criteria (Appendix 2): 11 review articles, five clinical studies, two case reports, two protocol papers and one primary biomedical research paper. The findings of these articles were analysed thematically, with the following topics extracted:
LDN mechanism of action in FM
LDN may improve symptoms in patients with FM by changing the processes of central and peripheral sensitisation.15 LDN in the range 1–5 mg/day operates beyond its typical role of opioid receptor antagonist and exerts immunomodulatory and anti-inflammatory effects through glial cells in the CNS and peripheral nervous system.15,18 LDN is thought to antagonise Toll-like receptor 4 activity in glial cells and consequently reduce activation of the inflammatory cascade and nociceptive system.15,18 An in vitro biochemical study on microglial cells has provided evidence that naltrexone is a neuroprotective substance that could be a promising novel treatment for various neuroinflammatory conditions.19 Another proposed analgesic mechanism is reactive upregulation of endogenous opioid production due to transient and partial opioid receptor blockade.20
Clinical studies of LDN in FM
LDN dosage, frequency and preparation
As indicated in Table 2, the total daily dose of LDN in the five included clinical studies ranged from 3 to 9 mg,21–25 with daily dosing of 4.5 mg being the most common. Bruun-Plesner et al explored the dose–response relationships of LDN for FM (using global improvement in FM symptoms to measure response) and estimated the ED50 and ED95 (effective dose for 50% and 95% of participants, respectively) to be 3.88 and 5.40 mg, respectively, concluding that 4.5 mg, as used in previous trials, is a reasonable dose.21 In four of the five studies, LDN was administered at night, changing to morning if there were significant vivid dreams or insomnia.21,23–25 All LDN was administered orally, and the preparation was generally a compounded capsule; however, water-dissolved naltrexone was used via syringe for dose titration in one study.22
|
Table 2. Clinical trials of low-dose naltrexone for the treatment of fibromyalgia
|
|
Author
|
Objective
|
Design
|
Sample size/population
|
Daily dose
|
Study duration
|
Notable outcome
|
|
Bruun-Plesner et al 202021
|
To explore dose–response relationships when treating FM with LDN
|
Single-blinded trial
|
n = 25 (excluding 2 withdrawn)
|
N/A
|
3 weeks
|
Authors estimated the ED50 to be 3.88 mg and the ED95 to be 5.40 mg. Based on these findings, a dose of 4.5 mg seems to be a reasonable test dose in FM patients, because it lies in the range between the ED50 and ED95. The primary endpoint was subjective evaluation of improvement of overall FM symptoms using a seven-point transition scale (PGI-I scale)
|
|
Jackson et al 202122
|
To identify what chronic opioid treatment does to pain tolerance as measured by the CPT, and to find an alternative effective treatment for chronic pain and FM
|
Prospective open-label case series
|
n =76 (55 with OIH; 21 with FM)
|
Up to 9 mg (4.5 mg bd)
|
Ranged from 36 to 60 days
|
Comparing initial and last CPT times, those with OIH more than quadrupled their pain tolerance, and those with FM doubled theirs. This improved pain tolerance for OIH and FM was statistically significant (P < 0.0001 and P = 0.003, respectively) and had a large effect size (r = 0.82 and r = 0.63, respectively)
|
|
Parkitny et al 201723
|
To test the hypothesis that LDN reduces inflammation in FM
|
Single-blind cross-over pilot trial
|
n = 8
|
4.5 mg
|
10 weeks
|
Significant reductions in the following inflammatory plasma markers vs baseline: IL-1β, IL-1Ra, IL-2, IL-4, IL-5, IL‑6, IL-10, IL‑12p40, IL-12p70, IL-15, IL‑17A, IL-27, IFN-α, TGF-α, TGF-β, TNF-α, and G-CSF
Significant reductions in FM-associated pain (15%), and overall symptoms (18%) measured using a VAS
|
|
Younger et al 200924
|
To test the effectiveness of LDN in treating the symptoms of FM
|
Single-blind cross-over pilot trial
|
n = 10
|
4.5 mg
|
14 weeks
|
Significant impact on daily pain (P = 0.001), highest pain (P = 0.005), fatigue (P = 0.008) and stress (P = 0.003) measured using a VAS
Significant reduction in VAS-measured pain threshold (P = 0.001)
FIQ scores reduced by 31.7% with drug vs 16.7% for placebo (P < 0.0005)
|
|
Younger et al 201325
|
To determine whether low dosages of naltrexone reduce FM severity vs the non-specific effects of placebo
|
Randomised placebo-controlled cross-over double-blinded study
|
n = 31
|
4.5 mg
|
20 weeks
|
Significant improvement in FIQ-measured pain (P = 0.016), satisfaction with life (P = 0.045) and mood (P = 0.039)
|
|
CPT, cold pressor test; ED50, median effective dose (the dose for 50% of the population to obtain the therapeutic effect); ED95, the dose required to achieve the desired effect in 95% of the population; FIQ, Fibromyalgia Impact Questionnaire; FM, fibromyalgia; G-CSF, granulocyte colony stimulating factor; IFN-α, interferon-α; IL, interleukin; IL-1Ra, interleukin-1 receptor antagonist; LDN, low-dose naltrexone; N/A, not available; OIH, opioid induced hyperalgesia; PGI-I, Patient Global Impression of Improvement; TGF, transforming growth factor; TNF-α, tumour necrosis factor-α; VAS, visual analogue scale.
|
Side effects
LDN was reported to be well tolerated in all five trials (Table 2).21–25 The pilot study done by Younger et al reported two individuals with vivid dreams and one individual who experienced transient nausea and insomnia for the first few nights.24 The recent dose–response study by Bruun-Plesner et al found gastrointestinal symptoms to be the most common side effects, including abdominal ache, diarrhoea, constipation and nausea.21 These side effects were common but generally graded mild and tolerable. In contrast with other pharmacological treatments of FM, LDN has a benign safety and side effect profile.26,27
Treatment effects and effect size
In all prospective clinical studies, LDN showed statistically significant positive effects on pain or pain tolerance measures.21–25 Other effects reported in case series included improved satisfaction with life, improved mood and decreased stress.6,14 A reduction in inflammatory markers was also noted.23
Study size and external validity
All included studies had a relatively low sample size (n = 8–31) with short periods of follow up (3–20 weeks).21–25 Despite the small sample sizes, P-values were generally low, indicating a low likelihood that observed differences were due to chance. Two protocol papers published in 2021 and 2022 were found, proposing studies with 100 and 120 participants and having a follow up of 12 weeks and 12 months, respectively.28,29
Discussion
Clinical implications
There is early evidence of the potential benefit of using of LDN in FM, with improvements in many areas of the syndrome, including pain, fatigue, sleep and cognitive symptoms. The effect sizes are such that one would expect clinically significant change. This, balanced with the comparatively benign safety profile of LDN, makes it a medication that may be worth trialling with patients with FM, especially if harmful medications may be reduced or ceased with its introduction.
Administration guidelines
Typical clinical dose administration from this review would resemble the following:
-
daily dosing, initially at bedtime but changing to morning if vivid dreams are problematic
-
titration regimen: 1.5 mg daily for the first week, 3.0 mg daily for the second week, then 4.5 mg for ongoing treatment if well tolerated (1.5-mg compounded capsules may be used for titration).
Side effects are usually mild and include gastrointestinal cramping and diarrhoea, as well as vivid dreams. LDN may be trialled while taking opioids, but the incidence of gastrointestinal side effects is likely to be greater due to enteric opioid receptor antagonism. LDN is unlikely to cause frank withdrawal due to the low dose. A trial duration of 3 months would be reasonable.
Other conditions
Other syndromes have much in common with FM, including chronic fatigue syndrome/myalgic encephalitis (CFS/ME) and long COVID (LC), with 30% of cases of CFS/ME and long COVID meeting the ACR diagnostic criteria for FM.16 There is emerging evidence for the use of LDN for the treatment of both CFS/ME and long COVID, which raises the possibility of a shared pathophysiology between these syndromes and FM.30,31
Future research
Although there is promising preliminary research, this review has demonstrated a lack of robustly constructed, large clinical studies, revealing only three prospective, randomised, blinded trials with sample sizes of 8, 10 and 31 patients.23–25 Further research with larger sample sizes would help define the expected effect size and number needed to treat. The authors are in the process of planning a randomised controlled cross-over trial with a sample size of 80 in order to add certainty to treatment guidelines.
Conclusion
Despite a lack of robust experimental evidence, preliminary trials and case series in FM show symptom improvement with LDN and a benign side effect profile. Further robustly constructed research with larger sample sizes is needed. These studies are in the planning/early execution stages and results should be available in 2024.