This article is part of a series of articles on infertility.
Pelvic inflammatory disease (PID) refers to the inflammatory pathology of the female upper reproductive tract that occurs in response to infection with microbial pathogens. This may present with clinical inflammatory illness, with symptoms of fever, pain, vaginal discharge and abnormal bleeding. Symptoms may also be subtle or even absent, leading to a delay in the diagnosis of subclinical infection.1 The true incidence of PID is uncertain due to difficulty in ascertaining and reporting a diagnosis.2 It is likely that women in developing countries are disproportionately affected by PID, and rates in Aboriginal and Torres Strait Islander communities have been reported to be as high as 32%.3
Pathogenesis
The ascension of microbes to the upper reproductive tract occurs as a result of disruption of the normal protective barriers of the lower reproductive tract by the causative pathogens (Figure 1). Initial infection of the cervix moves to involve the endocervical canal and breaks down the mucous plug barrier. Women are particularly susceptible during the mid-cycle, when the migration of pathogens may be facilitated by uterine peristalsis that facilitates sperm transport. This may be further compounded by the loss of the mucous plug at the time of menses and the retrograde spill of menstrual fluid into the pelvis.4
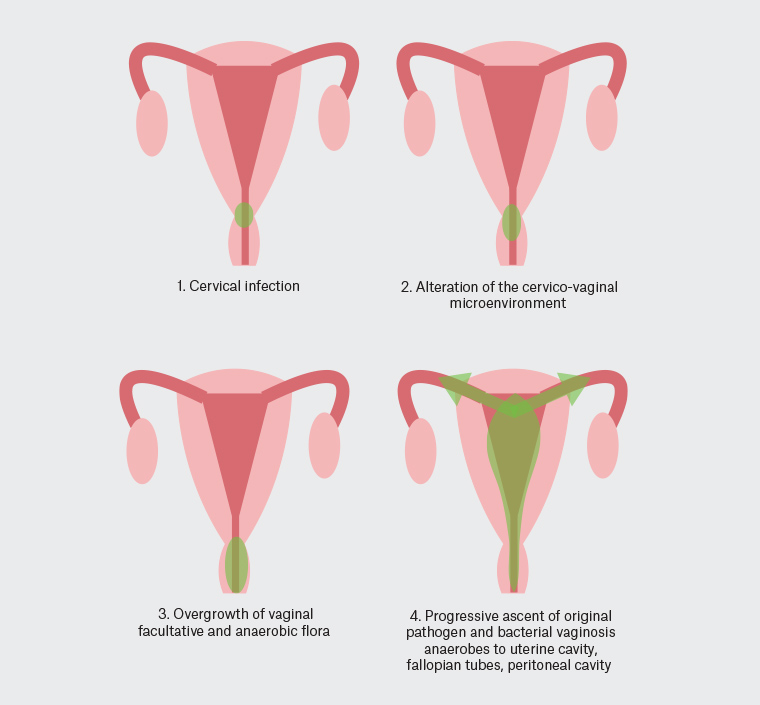
Figure 1. Pathogenesis of pelvic inflammatory disease.17
Chlamydia trachomatis and Neisseria gonorrhoea are commonly isolated during the diagnostic evaluation of approximately one-third to one-half of women presenting with PID.5 The Royal Australian College of General Practitioners (RACGP) Guidelines for Preventative Activities in General Practice recommend opportunistic screening for C. trachomatis in sexually active persons aged 15–19 years due to the prevalence and risk of complications in this cohort.6
After initial infection, it is common for a polymicrobial infection to develop from the transposition of vaginal facultative organisms, including those associated with bacterial vaginosis, and respiratory and gastrointestinal organisms that have colonised the lower genital tract. A list of commonly isolated pathogens is provided in Table 1.1
| Table 1. Causative pathogens in pelvic inflammatory disease |
| Cervical pathogens |
Neisseria gonorrhoea, Chlamydia trachomatis, Mycoplasma genitalium |
| Bacterial vaginosis pathogens |
Peptostreptococcus species, Bacteroides species, Atopobium species, Leptotrichia species, Clostridia species, Mycoplasma hominis, Ureaplasma urealyticum |
| Respiratory pathogens |
Haemophilus influenzae, Streptococcus pneumoniae, Group A streptococcus, Staphylococcus aureus |
| Enteric pathogens |
Escherichia coli, Bacteroides fragilis, Group B streptococci, Campylobacter species |
Clinical evaluation
The classical presentation of acute pelvic inflammatory disease is one of the abrupt onset of pain symptoms during or just following menses. Pain is most likely to be located in the lower abdomen in relation to the pelvic organs, but may be generalised in the setting of peritonism or even located in the upper abdomen in cases where there is perihepatic inflammation. Due to the significant heterogeneity in symptoms and presentation and the risk of complicated disease, the clinician must have a low threshold for suspecting the diagnosis. Clinical diagnosis is made by the finding of pelvic organ tenderness, comprising cervical motion tenderness, uterine tenderness on bimanual compression and/or tenderness in the adnexae, in combination with cervical inflammation, as evidenced by discharge, friability and/or a high white blood cell count on microscopic examination of the vaginal fluid.1
All patients presenting with symptoms potentially caused by PID should have high vaginal and endocervical swabs sent for microscopy, culture and polymerase chain reaction (PCR) testing. Endocervical samples are preferred to self-collected first-void urine samples due to superior detection rates. Elevated serum inflammatory marker concentrations may also be present.2 In cases of high diagnostic suspicion, it is reasonable to initiate antimicrobial treatment as per local guidelines pending culture results and antibiotic sensitivities. In approximately 20–30% of cases, no causative organism will be isolated; therefore, initiation of therapy is warranted on clinical grounds alone. Early and effective antibiotic treatment reduces the long-term morbidity of PID, with subsequent pregnancy rates two- to threefold higher in the modern antibiotic era.7 Consideration should be given to the possibility of concurrent pregnancy and broad testing for sexually transmissible infections, performed with the appropriate pretest counselling.
Long-term sequelae
The sequelae of PID are a major cause of reproductive morbidity in women of childbearing age, resulting in chronic pelvic pain, ectopic pregnancy and infertility. Despite the high rates of clinical response to antibiotic treatment, approximately 18% of women will report infertility, 0.6–2.0% will have an ectopic pregnancy and 30% will have chronic pelvic pain at three years after treatment.7 The risk of infertility increases in correlation with the degree of fallopian tube damage seen at laparoscopy, and may be as high as 30% in women with severe tubal damage. Recurrent infections are associated with a marked increase in the risk of infertility.8 In women with a history of PID, discussion of the long-term sequelae and the implications for fertility is an essential component of treatment and post-treatment counselling. Similarly, the risk of ectopic pregnancy and need for early pregnancy monitoring to site the pregnancy are important components of treatment counselling.
Tubal infertility
Incidence
Approximately 15% of couples will experience infertility.9 Tubal factors account for 25–35% of cases of female infertility, with PID being the causative factor in more than half. The risk of tubal infertility increases with recurrent PID and, after three episodes, more than 50% of women will have tubal dysfunction.10 Therefore, questioning regarding previous pelvic infections should form part of the initial interview in couples seeking preconception planning or trying to conceive. Guidelines for infertility assessment recommend evaluation of the infertile couple after 12 months of unprotected intercourse or 6 months where the female partner is aged >35 years.11 Just as this recognises the significant contribution of female age to female infertility, the history of previous PID would also warrant earlier assessment.
Evaluation
PID may result in proximal and/or distal disease of the fallopian tube. The presence of a hydrosalpinx, the degree of peritubular adhesions, the preservation of fimbriae and appearance of the endosalpinx are considered in the assessment of disease severity.12 There are multiple modalities for assessment of tubal patency.
Baseline pelvic ultrasound, a routine component of the fertility evaluation, may detect hydrosalpinges in women with severe tubal disease. A hysterosalpingogram may be performed with water- or oil-based radio-opaque contrast media and fluoroscopy to delineate the uterine cavity and the fill and spill of the fallopian tubes. This modality has a sensitivity and specificity of 65% and 83%, respectively. Sonohysterography uses ultrasound and water-based contrast with a sensitivity and specificity of 76% and 67%, respectively, for tubal patency.13 The gold standard for tubal assessment is chromotubation at laparoscopy. Chromotubation allows for the assessment of the pelvis in full, including coexisting pathology, evaluation of the tubal anatomy and relationship to the ovaries, and the detection of peritubal adhesions. Arguably, when the degree of suspicion for tubal disease is high, laparoscopy is the preferred method for assessing tubal function.
Management of tubal infertility
The ideal management for couples with tubal infertility is an individualised one, based on female age, ovarian reserve parameters, coexisting pelvic pathology and semen parameters, in addition to socioeconomic factors and patient preferences.
Treatments are site specific for proximal or distal disease. Transcervical tubal cannulation is rarely performed in Australia and, although resolution of tubal blockage is reported in 85% of cases, rates of re-occlusion are high (30%).14 Microsurgical tubocornual anastomosis excises the occluded proximal tubal portion, re-anastomosing the patent interstitial and distal tubal ends. Results from cohort and observational studies with small case numbers suggest ongoing pregnancies in approximately 48% of women after the procedure.14
Distal tubal disease is present in the greater proportion of women with tubal infertility. Salpingostomy and fimbrioplasty are potential surgical therapies to increase tubal function. Salpingostomy, incision of the diseased distal tube, is associated with subsequent pregnancy rates of approximately 30%, one-quarter of which will be ectopically sited.12 Fimbrioplasty, attempts to restore fimbrial function and oocyte capture by lysing adhesions around the fimbria and everting the fimbrial edges with macroscopic, microscopic or laparoscopic sutures.12 Although surgery for distal tubal disease offers a reasonable prospect for re-establishing tubal patency, it cannot reverse the ciliary damage of the tubal epithelium and architecture that results from the causative disease process.14
In vitro fertilisation (IVF) eliminates the role of the fallopian tube in successful conception and overcomes this obstacle in couples with tubal factor infertility. Controlled ovarian hyperstimulation with gonadotropins is followed by transvaginal oocyte collection and insemination in the IVF laboratory with partner or donor spermatozoa. Embryos are transferred to the uterus via a transcervical catheter. In all comers, the chance of successful conception with IVF treatment is approximately 30%; however, the most important determinant of IVF success, all factors otherwise being equal, is female age.15
No randomised trials to date have addressed the role of surgery compared with IVF for tubal factor infertility. In young women with normal ovarian reserve markers and mild tubal disease who prefer a primary surgical approach, primary surgery may be a reasonable approach with recourse to IVF treatment if this is unsuccessful in achieving pregnancy.12 In the presence of hydrosalpinges, tubal occlusion or salpingectomy is recommended prior to embryo transfer due to the deleterious effect on implantation and pregnancy rates.16
Conclusion
Tubal infertility is a not uncommon sequela of PID. The approach to management is individualised, based on patient factors, disease severity and patient preferences. Given the high incidence of long-term complications, public health initiatives to prevent sexually transmissible infections, as well early detection, diagnosis and treatment, are targets to reduce disease morbidity.