During pregnancy and postpartum, hypothyroidism is common, and its treatment with thyroxine (T4 ) may exacerbate the known problem of inadequate dairy intake during this period. Clear guidance would help rectify this.
Overt hypothyroidism occurs in 0.3–0.5% of pregnant women and subclinical hypothyroidism occurs in approximately 2–3% of pregnancies.1 International and national guidelines recommend T4 replacement for both ‘overt’ hypothyroidism (low free T4 , high thyroid-stimulating hormone [TSH]) and subclinical hypothyroidism (normal free T4 , TSH over the gestation-specific reference interval or >4 mU/L if unavailable).2,3
The prescription and dosing of T4 , as well as counselling as to correct administration, should be as per Therapeutic Goods Administration guidance: on an empty stomach, with no food for at least 30–60 minutes after the dose to optimise absorption.4 Because calcium supplements decrease the bioavailability of T4 ,4,5 these should be separated from T4 by several hours.4 A small study of 10 healthy euthyroid subjects found concurrent ingestion of cow’s milk and oral T4 resulted in a modestly lower area under the curve (8% less) and peak levels (7.8% less) of serum total T4 , compared to the same subjects without ingestion of cow’s milk.6 However, there are currently no data on the consumption of dairy products after the recommended 30-minute delay. A systematic review in 2021 did not identify any further studies examining the specific interaction between T4 and dairy.5
Adequate diary intake is important for maternal and neonatal health. The Australian dietary guidelines recommend 2.5 serves of dairy per day for women aged 19–50 years, including pregnant and breastfeeding women.7 Healthy Bones Australia recommends 1000 mg/day calcium, equating to four serves of dairy.8 Concerningly, according to a 2015 Australian study, only 22% of pregnant women consume 2.5 serves of dairy per day, with the median consumption being 1.9 serves (interquartile range 1.5–2.4 serves).9 Low dairy intake in pregnancy is associated with small-gestational-age neonates and spontaneous abortion,10 in addition to maternal bone fragility.8
Clinicians in the Alfred Health Endocrinology in Pregnancy Clinic observed a pattern of pregnant and postpartum women reporting they were advised not to consume dairy for two hours after T4 administration,11 possibly an extrapolation of advice regarding calcium supplementation. Because T4 is commonly taken in the morning and dairy products are usually eaten for breakfast, this advice has the potential to reduce calcium consumption.
The Australian Pharmaceutical Formulary (APF) guides the use of cautionary advisory labels (CALs) to accompany drugs, providing administration advice and precautions. We found that the content of APF CALs may have inadvertently contributed to inconsistent advice. Prior to the 2021 Australian Pharmaceutical Formulary Handbook,12 the recommended labelling of T4 included the statement: ‘Do not take dairy products, antacids or mineral supplements within two hours of each dose of this medicine’ (Label 4a).13 We wrote to main medical dispensing software companies and, in turn, the APF New Drugs Advisory Group, to advise of our clinical concern relevant to this recommendation, given the lack of evidence base and potential for harm. This resulted in the revision of the APF advice (print and digital) for T4 to remove the mention of ‘dairy products’.12
There is often a significant lag time between changes in guidelines and real-world practice. Anecdotally, we still encounter women receiving advice to avoid dairy for two hours after T4. Pharmacy labelling systems may provide standardised text for CALs, not reflecting updated APF recommendations. Therefore, accurate T4 labelling may depend on manual revision of pre-set text. Thus, pregnant and postpartum women taking T4 would benefit from careful counselling at the time of prescription and dispensing, conveying the following message: ‘After thyroxine administration, wait 30–60 minutes before eating anything and 2 hours before taking calcium-containing supplements’. Indeed, all patients taking T4 should be advised this way.
In summary, hypothyroidism requiring T4 replacement is common in pregnancy, and many women do not meet their recommended dairy intake. Prescribers should be aware that there are no specific concerns about dairy intake with T4 and it is not considered separate to generalised food intake (Box 1; Figure 1). Patients should be advised to separate their T4 tablets from food (including dairy products) and other medication by 30–60 minutes, and longer for calcium-containing supplements (delay 2 hours). We hope the correction of previously inaccurate labelling advice will remove a potential barrier to adequate dietary calcium intake in this at-risk population.
| Box 1. Considerations regarding thyroxine and dairy intake advice for pregnant and postpartum women |
- Maternal thyroxine requirements increase physiologically during pregnancy
- There is increased incidence of thyroid dysfunction, including hypothyroidism, during pregnancy and in the postpartum period
- A low proportion of women of childbearing age meet dairy intake recommendations
- Significant intake of dairy occurs in the morning for breakfast (eg milk, yoghurt, cheese); thyroxine is also frequently prescribed as a morning dose
- Inaccurate drug labelling (Cautionary Advisory Label 4a) of thyroxine boxes compounds the issue of inadequate dairy intake for women prescribed thyroxine replacement
- After thyroxine administration, only 30–60 minutes delay is needed before eating anything (including dairy products), whereas a two-hour delay is required before taking calcium-containing supplements
|
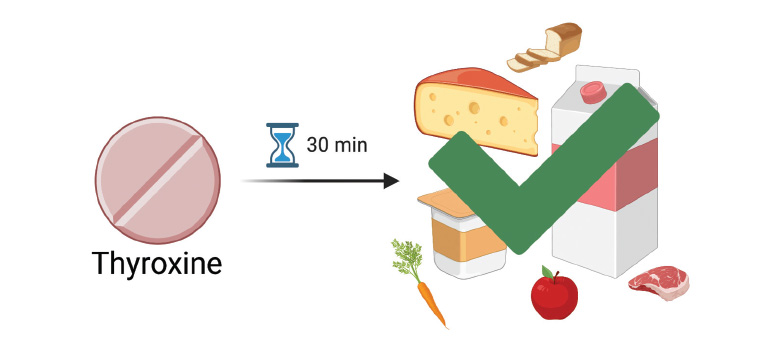
Figure 1. A delay of 30–60 minutes before eating any food (including dairy products) is recommended after thyroxine administration.