On 4 March 2022, Australia’s Acting Chief Medical Officer declared a communicable disease incident of national significance following the detection of Japanese encephalitis virus (JEV) in piggeries and the first cases in humans.1 The outbreak, initially thought to have begun in 2022, was subsequently genetically linked to a case detected in the Tiwi Islands in February 2021 that was considered to be the sentinel case.2
Since the start of 2021, 45 symptomatic human cases of Japanese encephalitis (JE) have now been identified in New South Wales, the Northern Territory, Queensland, South Australia and Victoria, including seven deaths (Table 1).3 JEV has also now been detected in more than 70 piggeries across these states, as well as in feral pigs in the Northern Territory and Queensland.4
| Table 1. Number of cases of Japanese encephalitis in Australia from 1 January 2021 up to 24 February 20231 |
| |
Confirmed casesA |
Probable casesB |
| New South Wales |
14 |
0 |
| Northern Territory |
2 |
0 |
| Queensland |
2 |
3 |
| South Australia |
6 |
4 |
| Victoria |
11 |
3 |
AConfirmed cases are defined on the basis of symptoms with definitive laboratory evidence.
BProbable cases are defined on the basis of symptoms with epidemiological linkage or suggestive laboratory findings. |
Prior to this outbreak, only five locally acquired cases of JE had been detected in Australia, including two deaths (Figure 1). All cases were restricted to northern Australia (the Torres Strait and Cape York Peninsula ) and no cases had been detected since 1998. Overseas-acquired cases of JEV have been sporadically detected in Australia.5,6
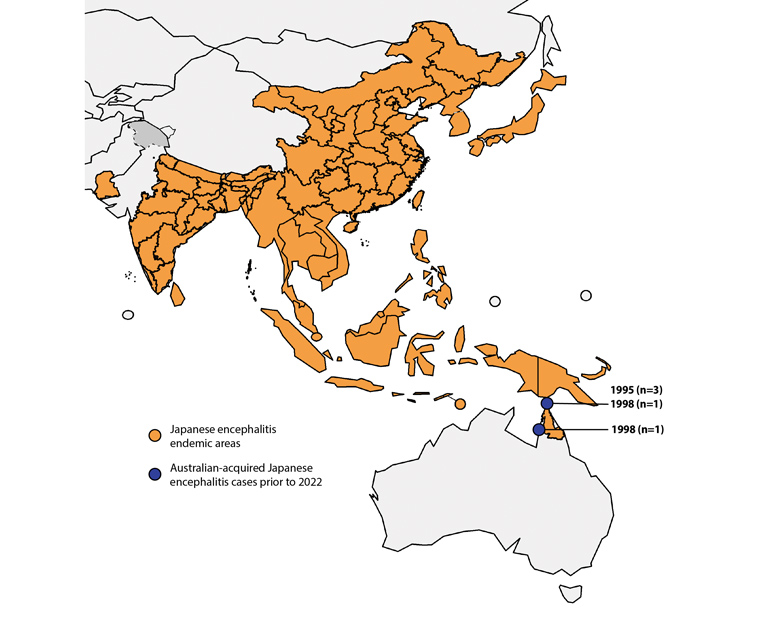 Figure 1. Distribution of locally acquired Japanese encephalitis cases in Australia prior to 2021.5,28
Figure 1. Distribution of locally acquired Japanese encephalitis cases in Australia prior to 2021.5,28
This is an adaptation of an original work “Map: Japanese encephalitis, countries or areas at risk” by the World Health Organization (WHO; 2018) published under Creative Commons licence CC BY-NC-SA 3.0 IGO. This adaptation was not created by the WHO. The WHO is not responsible for the content or accuracy of this adaptation. The original edition shall be the binding and authentic edition.29
The emergence of JEV in southern Australia represents a marked change in epidemiology. Altered environmental conditions because of climate change have been associated with changes in JEV distribution,7–9 and appear to be an important driver of this evolving epidemiology within Australia.10
Virus and transmission cycle
JEV is a zoonotic vector-borne single-stranded RNA virus of the family Flaviviridae, which also includes dengue, Murray Valley encephalitis, West Nile virus (Kunjin) and zika.11,12 There are five genotypes of JEV, all of which belong to the same serotype and have similar virulence.12,13 The recent Australian outbreak was identified to be genotype IV, a previously uncommon lineage that has been detected in China, Indonesia, Malaysia and Papua New Guinea.6,14 This genotype is different from earlier detections in Australia.5
JEV can infect several animal hosts, of which wild wading birds (herons and egrets; a natural reservoir) and pigs are the most important for the maintenance and spread of the virus. Transmission of JEV between animal hosts and humans occurs via mosquitoes, especially Culex species.15 In Australia, the major vector, Culex annulirostris,15 can travel several kilometres per day.16 Pigs act as an amplifying host and are important in the infection of humans. Once infected with JEV, pigs achieve a high level of viraemia lasting for 3–5 days that enables vector-borne onward transmission of the virus (Figure 2). Pigs are often farmed in large populations in close proximity to humans, with regular herd turnover ensuring persistent susceptibility.17 However, JEV transmission has been maintained in areas where pigs have been removed. Feral pigs are also thought to play an important role in the spread of the virus.17,18
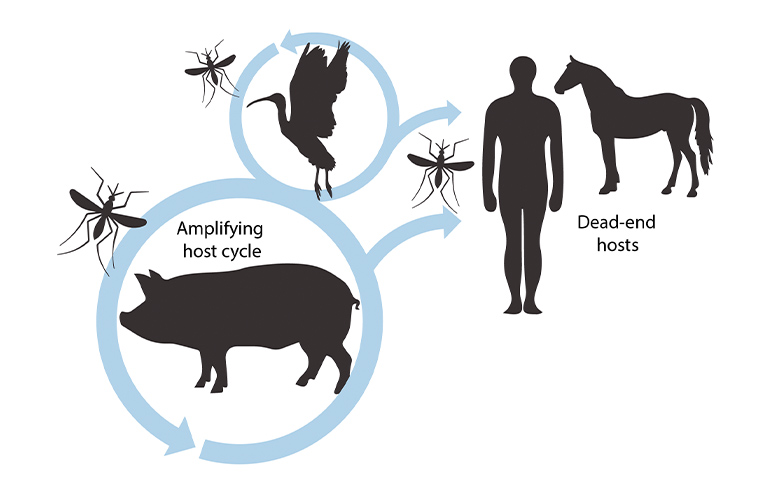
Figure 2. Transmission cycle of Japanese encephalitis virus.
Many animal species (eg cows, horses, dogs, chickens) can be infected with JEV, but only develop low levels of viraemia. These animals are not thought to be able to infect mosquitoes and contribute to onward transmission, and are termed ‘dead-end hosts’. Similarly, humans are considered dead-end hosts and cannot transmit the virus if infected.17,19 Transmission to mosquitoes from flying foxes in Australia has recently been described, indicating the potential role of other species in the transmission cycle.19 Most animals do not develop symptoms, but horses can develop fatal encephalitis and infected pigs can experience reproductive disease (increased rates of abortion, neurologically affected piglets and infertility in boars).17,20
Epidemiology
JE is one of the most important causes of infective encephalitis globally.21 JEV is endemic across most of Asia and the Western Pacific, with 3 billion people at risk (Figure 1).13 The vast majority of those infected with JEV remain asymptomatic, with the rate of symptomatic illness generally considered to be <1%.11 However, estimates of the proportion of those experiencing symptomatic disease vary significantly, from 1 in 25 to 1 in 1000, and are likely to be higher in non-endemic populations.11
Estimating the global burden of JE is challenging because many endemic countries have limited surveillance and diagnostic capacity.13,21 Recent modelling estimated that, in 2015, there were 100,308 cases of JE globally (95% confidence interval [CI] 61,720, 157,522) and 25,125 deaths (95% CI 14,550, 46,031).21
Cases of JE predominantly, although not exclusively, occur in rural areas, especially in proximity to rice cultivation, which provides a favourable environment for Culex mosquitoes, and pig farms.22 JE can affect people of any age, especially in non-endemic countries, where there is greater population susceptibility. In endemic regions, JE tends to have a higher incidence in children aged 0–14 years (estimated 5.4 cases per 100, 000) than in those aged ≥15 years (0.6 cases per 100,000), due to adults having gained immunity from subclinical infection in early life.23 However, even in endemic regions, there is substantial variation in the age distribution of cases between countries.13,22 In tropical regions, JE transmission occurs year round, with a peak during the wet season when mosquito density is highest. In temperate and subtropical regions, epidemics tend to occur in warmer months.13,22
Extensive research has linked the distribution of arboviruses with temperature and rainfall, and has highlighted the risk of expansion into new areas, with immunologically naïve populations, as a result of climate change.7–10 Beyond climate change, global expansion of JE has been driven by increases in travel, rice cultivation, pig farming and the proximity of these agricultural practices to population centres as they expand and require increased food supply.22
The outbreak in Australia was preceded by several months of heavy rainfall associated with La Niña, which led to the formation of inland wetlands, the migration of wading birds to southern regions and the proliferation of mosquitoes.6,10 These weather conditions, under the effects of climate change, are believed to be key drivers of the emergence of JEV in southern Australia and other temperate regions.9,10
Presentation
The main risk factors for JE are exposure to mosquitoes in areas where JEV is present.3,12 The incubation period for JEV is 5–15 days.11,24 The early clinical phase usually begins with a non-specific febrile illness lasting several days, involving coryza, gastrointestinal symptoms and sometimes a rash (Figure 3). The onset of neurological symptoms may be abrupt and progress rapidly. Symptoms include headache, irritability, agitation, confusion, drowsiness and coma.11,12,24 Features typical of meningitis may also be present, as part of the spectrum of meningoencephalitis.11,25 Disordered movement is a characteristic feature of JE. Parkinsonian features (mask-like facies, cogwheel rigidity and pill-rolling tremor) may be present, as well as generalised rigidity, dystonias and choreoathetosis. Acute flaccid paralysis may also occur.11,12,24 Seizures are especially frequent in children, occurring in up to 85% of cases (compared with 10% in adult cases). Seizures can be focal or generalised. Persistent seizure activity is an indicator of raised intracranial pressure and is associated with poor outcomes.11
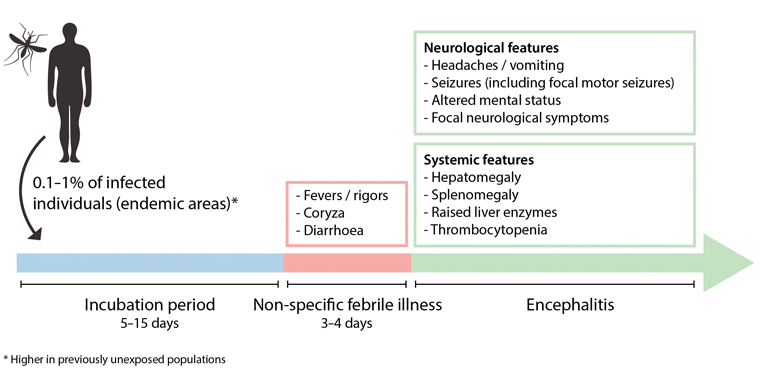 Figure 3. Clinical manifestations of Japanese encephalitis in humans.
Figure 3. Clinical manifestations of Japanese encephalitis in humans.
The average reported case fatality for JE is 18%, but estimates vary from 5% to 50%.12 Approximately half of those who survive experience permanent neurological deficits, including physical, psychological and cognitive impairments.12 Morbidity and mortality may be higher in children and the elderly.13
Investigations
JE should be suspected in cases of encephalitis, meningitis or flaccid paralysis.11,24,25 Prior to the 2022 outbreak in Australia, JE was primarily a consideration in returned travellers and residents of the Torres Strait.6 However, it is now prudent to consider JE more widely, especially for those who have had exposure to mosquitoes and are from areas with known case detections.
Diagnosis of JEV is challenging due to limitations in the sensitivity and specificity of diagnostic tests. Polymerase chain reaction (PCR) tests for JEV can be performed on cerebrospinal fluid (CSF) or other samples (whole blood, urine, serum) and, if positive, are confirmatory. The clinical sensitivity of PCR tests is limited by the fact that viraemia is transient and low level, meaning that serological techniques play an important role in the diagnosis of JE.26
The detection of JEV IgM in CSF is considered to be the gold standard for diagnosis, and, if present, is consistent with a diagnosis of JE.25,26 A diagnosis of JE can also be confirmed by the demonstration of seroconversion or a rising IgG titre on serial blood samples. The interpretation of serological testing is complicated by the fact that antibodies against JE and other flaviviruses are highly cross-reactive. As such, testing for other flaviviruses (i.e. dengue, Murray Valley encephalitis and Kunjin) may need to be performed in parallel. The presence of anti-JEV antibodies may reflect recent or historical exposure, vaccination or exposure to a different flavivirus. Thus, the interpretation of JE serology results requires information about travel and vaccination history, with an acute sample and convalescent sample usually needed. Testing should be discussed with the relevant laboratory or infectious diseases service before ordering.26
Although not diagnostic, abnormalities may be seen on magnetic resonance imaging, whereby JE causes parenchymal inflammation, which classically affects the bilateral thalami and can also involve the basal ganglia and brain stem.11,25
Investigations should also seek to identify alternative causes of meningoencephalitis and/or flaccid paralysis, as reviewed in detail elsewhere.25
Treatment
Presentations of encephalitis and meningitis are medical emergencies that require urgent assessment and management, including general resuscitative measures. Undifferentiated patients should receive empiric antimicrobial therapy, which, in the case of potential bacterial meningitis, should not be delayed to facilitate investigations.25 Patients presenting to primary care should be transferred to a hospital setting.
There are no specific drug treatments that have been demonstrated to have efficacy in treating JE, and management is supportive.12 This includes controlling seizures and managing raised intracranial pressure to minimise neurological damage.25 Those with permanent neurological sequelae are likely to require long-term follow up.
JE has been a nationally notifiable disease in Australia since 2001, and all confirmed cases must be notified to the relevant jurisdictional health authority.6,26
Prevention
Vaccines against JEV are available and are a key strategy for the prevention of illness.6,13 It is estimated that from 2010 to 2015, vaccination has prevented 307,774 cases (95% CI 167,442, 509,583) and 74,769 deaths (95% CI 37,837, 129,028) globally.21 Personal protective measures to limit mosquito exposure are also important and should include: limiting time outdoors during peak mosquito activity; wearing long, loose-fitting clothing; regularly applying effective mosquito repellent; removing water-holding containers where mosquitoes can breed; and ensuring entries to accommodation are covered with mosquito nets or screens.3
Two effective JEV vaccines are available for use in Australia.27 One of these vaccines is Imojev (Sanofi-Aventis Australia), a live-attenuated vaccine given as a single-dose vaccine via the subcutaneous route that cannot be given to people who are immunocompromised, pregnant or breastfeeding.13,27 The primary course of Imojev is a single subcutaneous dose administered to those aged ≥9 months. A second dose is recommended 12–24 months later for those aged <18 years at ongoing risk of exposure. No boosters are currently recommended for adults.27
The other vaccine available for use in Australia is JEspect (Sequiris), an inactivated vaccine provided in two doses via the intramuscular route; these doses are usually administered 28 days apart, but adults can be given an accelerated 7-day course if they are at imminent risk of exposure. Adults are recommended to have a booster 1–2 years after their primary course, if they face ongoing exposure risk. There are no data to inform booster dose recommendations in individuals aged <18 years, but these can be considered in children requiring sustained protection.27 Pregnant women can receive JEspect if they are at risk of acquiring JE in pregnancy, which has been associated with miscarriage in first and second trimesters.27 Both vaccines have been shown to induce neutralising antibodies in a high proportion of vaccinated individuals.13,27 Individuals who have been vaccinated with earlier-generation mouse brain-derived vaccines can receive a booster with either Imojev or JEspect if they face ongoing exposure risk.27
In Australia, recommendation for routine vaccination was previously limited to laboratory workers who may be exposed to JEV, travellers spending ≥1 month in endemic areas and individuals who live or work in the Torres Strait. Following the 2022 outbreak, vaccination has also been prioritised for travellers spending <1 month in endemic areas undertaking activities with increased risk of mosquito exposure, individuals with direct exposure or in close proximity to pigs and mosquitoes, those with high-level occupational exposures, as well as specified priority groups identified by state and territory health authorities.3,27,28 In response to the outbreak, the Australian Government purchased 130,000 JE vaccine doses for high-priority groups.6 A primary course for JE vaccination usually costs approximately $150–$300. In several jurisdictions, priority groups are eligible for free immunisation, and clinicians should contact their local public health service for details. It is likely that vaccination recommendations and public health measures will be updated in the coming months and years in response to updated surveillance information regarding the ongoing evolution of JE in Australia. It will be important for general practitioners (GPs) to monitor for updated advice from state and territory health departments. Health alerts are also issued from the Australian Government Department of Health and Aged Care for disease outbreaks affecting multiple jurisdictions, as was the case for JE in 2022, and these include advice from the Communicable Diseases Network of Australia.1
Various other control measures have been implemented for JE, including the vaccination of pigs, the separation of pig farms from human settlements and vector control with chemical agents or environmental management. However, there is limited evidence to support the effectiveness of these measures in reducing JE disease burden.13
Implications for practice
Although local environmental factors remain important, the advent of a widespread JE outbreak in Australia is a clear example of the potential for climate change to disrupt the epidemiology of vector-borne diseases. The established disease burden in the Asia-Pacific means that GPs should be alerted to the potential of rising case numbers in Australia, especially during the summer months in temperate and subtropical regions, and all year round in tropical regions. Familiarity with JE is now important for Australian GPs, especially for those practicing in rural and regional areas where the presence of JEV has been detected.