Respiratory disease is a common reason for presentation to primary care in Australia.1 Approximately 11.2% of all Australians live with asthma;2 14.5% of those aged >40 years live with chronic obstructive pulmonary disease (COPD)3 with additional illness burden provided by other respiratory diseases. Spirometry is essential for the diagnosis and management of many respiratory diseases, and is specifically mentioned in both the Australian asthma handbook4 and the Australian and New Zealand COPD-X guidelines.5 However, spirometry remains underutilised in primary care settings in Australia and overseas.6–9
A study undertaken for the Australian Institute of Health and Welfare found that 81.6% of included patients on medications for COPD did not have lung function testing undertaken in either the 12 months prior to or after inhaler initiation.10 In the same study, only 26.2% of patients with a diagnosis of asthma had evidence of lung function testing in the recent three-year period.10 Another Australian study found that half the patients reporting a diagnosis of COPD were probably misdiagnosed when spirometry was undertaken,11 and a Canadian study reported that misdiagnosis of COPD was fivefold more common than spirometrically confirmed COPD.12
Primary healthcare in Australia is structured around private general practice. General practitioners (GPs) are largely funded through fee-for-service payments,13 which are rebated by the national health insurance scheme, Medicare. General practices might have their own equipment for spirometry or might refer to another provider. In either case, the service might be fully reimbursed by Medicare or the patient might incur a gap payment.
The barriers to patients undergoing spirometry in Australian primary care are not well understood. Thus, this systematic review was performed to identify the barriers to correct spirometry reported in studies of Australian general practice.
Methods
Search strategy
We followed the PRISMA guidelines in undertaking and reporting this systematic review.14 We searched the MEDLINE, EMBASE, CINAHL, Scopus, PubMed and Google Scholar databases for all studies investigating barriers to correct spirometry use. The following search terms were used: ‘primary health care’, ‘family physicians’, ‘family practice’, ‘general practice’, ‘primary care’, ‘Australia’ and ‘spirometry’. We were helped by a professional medical librarian in developing the search strategy. The search was limited to studies published between 2000 and 12 May 2022.
The search strategy yielded 229 abstracts for initial consideration. The abstracts were screened for relevance by RL. Potentially relevant papers were retrieved and the full text was assessed by two independent reviewers (RL, TS). Disagreements were resolved by discussion with a third researcher (TU). A full description of the search strategy is provided in Appendix 1.
Inclusion criteria
The review was limited to articles published in English and reporting on studies of Australian general practice. Studies identifying one or more potential barriers to correct spirometry were included. All trial types, including randomised controlled trials, cohort studies, surveys and qualitative studies, were eligible. Review articles, systematic reviews and manuscripts not reporting original data were excluded.
Identification and presentation of reported barriers
No scheme for categorising barriers to spirometry was found. Two researchers (RL, TS) read the full text of potentially relevant studies. Potential barriers to spirometry in each study were independently noted. The two researchers (RL, TS) met to organise and define barriers based on the issues identified from the studies to consolidate both quantitative findings and qualitative responses. Following this, all three researchers met to discuss the barriers identified and to formalise the definitions presented here and described in Table 1. In this process, some barriers (eg ‘cost’ and ‘time’) were easy to identify and define, whereas others (eg ‘limited clinical utility’) emerged during discussions between the researchers. An iterative process of thoroughly reading the included studies, discussing the barriers raised and defining each barrier was undertaken by all three researchers. Once all barriers had been identified and defined, they were grouped into clinician, practice and patient issues, as reported in Table 1. The inclusion of all authors in this process minimised the risk of bias in the analysis.
| Table 1. Identified barriers to spirometry in Australian primary care |
| Barrier grouping |
Barrier |
Definition |
| Clinician issues |
Limited clinical utility |
Expressed a belief that spirometry has little clinical benefit and/or utility in the diagnosis of respiratory disease, and/or a preference to treat clinically rather than use spirometry for diagnosis |
| Confidence |
Expressed a lack of confidence in the role of spirometry or in interpreting spirometry |
| Interpretation skills |
Inaccurate interpretation of spirometry results when externally assessed by spirometry experts |
| Practice issues |
Time |
Lack of time to perform a spirometry test |
| Cost |
High cost of owning a spirometer
and/or performing spirometry relative to time taken and available reimbursement |
| Lack of trained staff |
Lack of staff who can perform the test |
| Poor availability |
Lack of spirometry onsite and/or reasonable offsite option able to undertake quality spirometry |
| Spirometry technique and calibration |
Poor spirometry technique when externally assessed by spirometry experts and/or spirometer poorly calibrated or uncalibrated |
| Patient issues |
Patient attendance |
Patient unwillingness or difficulty attending for spirometry |
An analysis of the risk of bias for each individual study was not undertaken. Because many of the barriers are reported in qualitative studies or substudies, a narrative metasynthesis approach was taken to analyse and present the findings.
Results
Literature search
Figure 1 outlines the results of the study search and selection process as per the PRISMA guidelines. The search strategy resulted in an initial yield of 390 references. The titles and abstracts were reviewed, with the full text of 210 articles subsequently retrieved and examined. Eleven articles were included in the final systematic review, comprising three randomised controlled trials15 (two with qualitative substudies16,17), one cohort study,18 two surveys19,20 and five qualitative studies.21–25 As indicated in Figure 1, no studies meeting the inclusion criteria were subsequently excluded. Table 2 summarises the included studies.
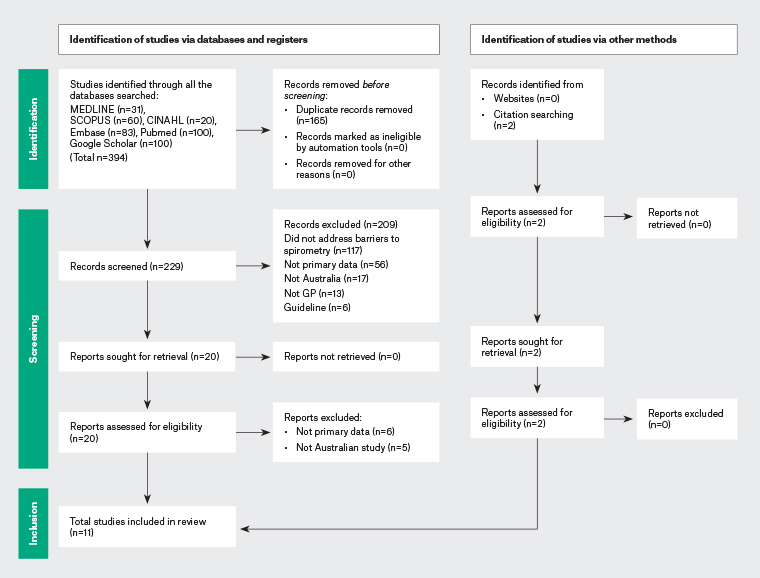
Figure 1. PRISMA diagram showing the identification of studies via databases, registers and other methods. Click here to enlarge
| Table 2. Included studies |
| Study |
Study type |
Study aim |
Population |
Overview of methods |
Relevant conclusions |
| Abramson et al 201216,A |
RCT with qualitative substudy: Individual telephone interviews |
Qualitative component: To determine doctors’ perceptions of spirometry |
31 general practices in Melbourne
The number of GPs interviewed not stated |
- In-depth individual telephone interviews with a sample of GPs
- Thematic analysis
|
- Limited clinical utility: Most GPs reported that spirometry was only necessary when they struggled with diagnosis or felt that the diagnosis was ‘more complicated’ or needed diagnostic confirmation
- Time: GPs reported time taken to undertake spirometry, as well as time to maintain equipment, as a barrier
- Cost: The cost of the spirometer and low reimbursement for undertaking spirometry were cited as barriers
|
| Borg et al 201018 |
Cohort study |
To determine whether spirometry training provides sufficient skill to produce valid results and whether follow-up training improves test validity |
15 nurses or physiotherapists (breakdown not stated) in Melbourne |
- Nurses or physiotherapists received 14 hours of training
- Technique reviewed at five, seven and nine months (assessed for adherence to ATS standards)
- Further education was provided after five and seven months
|
- Technique: ATS criteria not consistently met, although the proportion of tests meeting criteria improved over time with additional training
|
| Bunker et al 200915 |
RCT |
To assess the effectiveness, feasibility and acceptability of COPD case finding by PN |
Patients from four general practices in western Sydney |
- Patients randomised to receive invitation for spirometry or usual care
- PN interpretation of spirometry compared to investigator interpretation
|
- Interpretation: PNs correctly identified 10 of 16 patients as having COPD; however, they incorrectly labelled a further six patients with non-obstructive spirometry results as having COPD
- Patient attendance: Only 19.5% of patients accepted the invitation to attend for spirometry
|
| Dennis et al 201021 |
Qualitative: Focus groups |
To explore difficulties faced by GPs in making a diagnosis of asthma in adults |
18 GPs in south-west Sydney |
- Three one-hour focus groups using topic guide
- No qualitative analysis model stated
|
- Limited clinical utility: GPs reported being aware of the need for spirometry, but reported it was rarely performed and they were not confident of its role in diagnosis
- Confidence: Lack of confidence in the use of spirometry to diagnose asthma
- Time/patient factors: Time to undertake the tests and to persuade patients to engage in spirometry/follow-up visits
|
| Dennis et al 201722 |
Qualitative |
To explore implementation of a case finding/management COPD intervention |
GPs, PNs and patients involved in a cluster randomised controlled trial |
- Interviews with GPs and PNs coded according to a theoretical framework
- Patient interviews coded by thematic analysis
|
- Confidence: Some PNs described lacking confidence in interpreting spirometry; other PNs described needing to help GPs interpret spirometry
- Time: Time was a barrier to the use of spirometry
- Staff: Turnover led to skills in spirometry developed as part of the study being lost
|
| Goeman et al 200523 |
Qualitative: Structured discussion group |
To determine what GPs thought was needed to achieve best outcomes in people with asthma |
49 GPs (mixture of urban and rural) |
- Six groups were interviewed using a structured group interview process (nominal group technique)
|
- Confidence: Some GPs reported discomfort or difficulties interpreting spirometry
- Availability: Few general practices had access to a spirometer
|
| Hansen et al 201624,B |
Qualitative: Focus groups and individual interviews |
To explore the impact of whole-person, patient-centred care on how GPs engage with guidelines |
19 GPs in Tasmania |
- Focus groups and interviews with GPs regarding diagnosis of COPD and the use of guidelines
- No qualitative analysis model stated
|
- Limited clinical utility: GPs sometimes preferred to diagnose COPD based on clinical symptoms rather than spirometry
- The recommendation in guidelines to use spirometry would require a change in usual practice
|
| Johns et al 200619 |
Questionnaire by email and/or fax with telephone follow-up |
To determine the availability of spirometry and the level of spirometry training in general practice throughout Australia |
5976 general practices throughout Australia |
- Email/faxed survey sent to 5976 general practices
- Follow-up telephone survey of non-responders to detect whether initial response was biased to practices with spirometers
- Fax/email response rate: 19.6%
- 160 practices in telephone survey; 73.8% response rate
|
- Limited clinical utility: Spirometry not useful (5.5%)
- Confidence: Lacked confidence in interpreting results (17.9%)
- Time: Insufficient time (21.1%)
- Cost: Insufficient remuneration (32.8%)
- Staff: Did not employ a practice nurse (22.6%)
- Availability: Reasons for not owning a spirometer included equipment cost (53.3%)
- Calibration: Spirometer accuracy was never checked using a 3-L syringe (77.8%); did not test a healthy subject for quality control (40%)
|
| Liang et al 201720,C |
Survey |
To describe the availability of resources within general practices to enable interdisciplinary management of COPD |
41 practices recruited into a cluster randomised trial |
- Survey
- No further information available
|
- Staff: Few practices (30%) had staff who had recently undergone training in spirometry; fewer still (12%) had no staff trained in its use
- Availability: Minority (38%) of practices owned a spirometer
|
| Walters et al 200525 |
Qualitative: Focus groups and individual interviews |
To investigate the use of and attitudes to spirometry for COPD |
16 GPs and 38 patients in Hobart, Tas |
- Focus groups and semistructured interviews with GPs and patients
- No qualitative analysis model stated
|
- Limited clinical utility: Preference to manage respiratory disease with a trial of treatment rather than spirometry
- Confidence: Low confidence in interpreting spirometry
- Time: Time waiting for repeat spirometry after bronchodilator
- Availability: Lack of access to a well-maintained spirometer
- Staff: Lack of expertise in undertaking spirometry
- Patient attendance: Increased cost to patients for longer consultations; patient reluctance to attend a referral centre for spirometry
|
| Walters et al 200817 |
RCT comparing trained nurses undertaking spirometry to usual care
Qualitative substudy: Focus groups |
To compare the effects of opportunistic spirometry by visiting trained nurses with usual care on spirometry uptake
Qualitative component: Specific aim not stated |
Eight GP practices in southern Tasmania
28 GPs involved in focus groups |
- A six-month qualitative/quantitative cluster randomised study
- Outcomes were: Spirometry uptake and quality; new diagnoses of COPD; GPs’ experiences of spirometry
- No qualitative analysis model stated
|
- Limited clinical utility: Emphasis on clinical diagnosis rather than spirometry
- Confidence: GPs reported wanting assistance interpreting spirometry
- Interpretation: Small number of patients diagnosed with COPD despite having non-obstructive spirometry
- Time: GPs lack time to perform good-quality spirometry. Also, patient time to undertake spirometry an issue
- Cost: Cost a disincentive without appropriate funding
- Staff: PN seen as essential to perform spirometry
- Technique: Good-quality spirometry reported in 76% and 44% of tests in the intervention (who had received training in spirometry) and control (no training) arms, respectively (P=0.0001).
- Patient attendance: 32% of patients approached in the intervention group declined spirometry, often due to feeling unwell or time; recall system to undertake spirometry seen as burdensome
|
APart of a wider RCT. Only relevant aspects of the study are summarised here.
BThe study might contain data that have been reported in other studies in this systematic review (see the Methods section of this reference for details).
CAbstract.
ATS, American Thoracic Society; COPD, chronic obstructive pulmonary disease; GP, general practitioner; PN, practice nurse; RCT, randomised control trial. |
Barriers identified
The barriers identified fell into three categories: clinician, practice and patient barriers. Each of these is described in more detail below. Figure 2 shows the number of studies in each group following categorisation of the identified barriers.
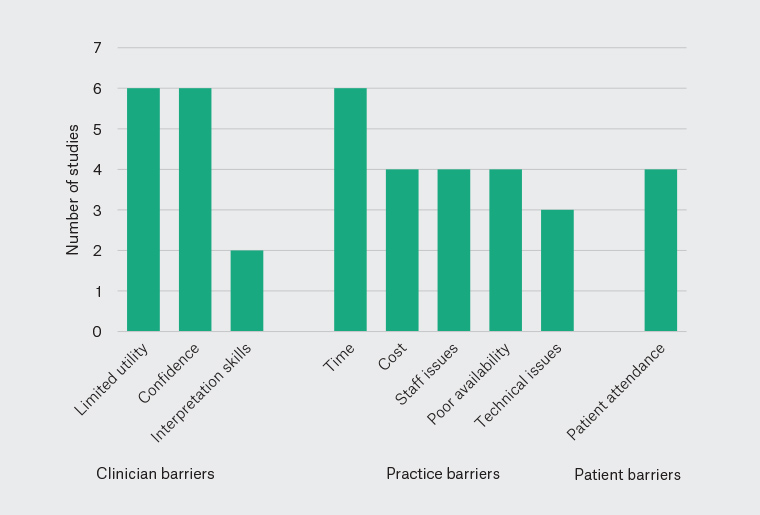
Figure 2. Number of studies reporting each of the identified barriers to spirometry in Australian general practice.
Clinician barriers
Limited clinical utility of spirometry
Six studies reported clinicians’ views about the clinical utility of spirometry.16,17,19,21,24,25 These studies reported a view that spirometry results had little or no clinical utility or that GPs preferred diagnosing COPD or asthma based on the patient’s clinical presentation, often over a period of time and/or a trial of treatment.16,17,19,21,24,25 One study suggested that undertaking spirometry as recommended in guidelines would require a change in practice.24 This view of spirometry persisted despite an understanding by the GPs themselves, as noted in some studies, regarding the importance of spirometry in respiratory disease.16,17,21
Confidence
Six studies were assessed that described a lack of confidence around spirometry.17,19,21–23,25 One study described GPs having a general lack of confidence undertaking and interpreting spirometry.21 The other five studies described GPs as lacking confidence in interpreting spirometry and/or wanting assistance in this task.17,19,22,23,25 In one study, which also included practice nurses, this lack of confidence persisted for some participants despite ongoing training and support provided by a respiratory scientist.22
Interpretation skills
Two studies demonstrated incorrect interpretation of spirometry by GPs17 or practice nurses.15 Both these studies reported that patients were diagnosed as having COPD despite spirometry that did not support this diagnosis. Concerningly, both studies had involved training of GPs in the interpretation of spirometry. Computerised support or external expert assistance to help with the reporting of spirometry was suggested by GPs in one study to overcome this barrier.17
Practice barriers
Time
Six studies also noted time as a barrier to undertaking spirometry.16,17,19,21,22,25 The time required for the process of advising patients of the need for spirometry, explaining and the procedure, conducting the test, waiting for the action of bronchodilators, repeating the test and interpreting the results was reported as a barrier to spirometry.
Cost
Four studies reported the cost of spirometers, undertaking spirometry and insufficient Medicare rebates as issues.16,17,19,25 Both the cost of consumables and the staff time required to perform spirometry were considered to be issues.
Staff issues
Four studies referred to a lack of trained staff to perform spirometry as a barrier.17,19,20,25 One study noted a high turnover of trained practice nurses and the impact this had on staff availability to undertake and interpret spirometry.22
Poor availability
Four studies highlighted that practices either lacked a spirometer onsite or did not have an accessible spirometry service to refer their patients to.19,20,23,25
Technical issues
Two studies demonstrated poor spirometry technique despite training in undertaking spirometry.17,18 Practice spirometry was compared to American Thoracic Society standards and found to not meet acceptability standards in terms of reproducibility and accuracy, despite initial training programs and ongoing training over several months.18 Spirometer calibration was noted to be an issue in one study, with deficiencies in both the frequency and technique of calibration reported.19
Patient issues
Patient attendance
Four studies suggested patient attendance for the purpose of undertaking spirometry is an issue.15,17,22,25 Two of these studies invited patients to attend for spirometry and reported acceptance rates of 19.5%15 and 68%.17 The other two studies reported a perception on the part of GPs that patients were reluctant to attend appointments solely for spirometry testing.22,25
Discussion
Summary of main results
This systematic review identified three categories of barriers to correct spirometry in Australian general practice. Clinician issues included a perceived lack of clinical utility of spirometry and low confidence in performing and/or interpreting spirometry. Practice issues included issues related to time, cost, staff, spirometer availability and maintenance, as well as the ability to undertake reliable spirometry. Patient issues were related to patient attendance.
The view among GPs that spirometry was of limited clinical utility was a frequent and unexpected barrier given that many GPs appeared to also recognise the importance of spirometry in diagnosing and managing respiratory disease.16,17,21 The importance of this finding is that it implies education in undertaking spirometry alone will be insufficient to increase the use of spirometry in general practice. Evidence suggests that the symptom-based approach to diagnosis is unreliable, with a recent Australian study finding that less than half the participants reporting COPD do not have obstructive spirometry when tested.11 Of note, that study also found that 6.9% of a population sample had undiagnosed COPD.11 A Canadian study focused on asthma found that 33% of participants did not have the diagnosis after exhaustive testing.26 This suggests that both under- and overdiagnosis are issues in the care of patients with COPD and asthma, with clear implications for harm related to untreated disease as well as the inappropriate prescription of medications, with concomitant cost and side effect issues. At least some degree of cultural/practice change will be necessary to increase the use of spirometry to avoid these potential harms.
It might be that there are broader issues than spirometry, with one paper suggesting GP attitudes to COPD more generally might play a part.27 In addition, a recent paper from a single Australian centre highlighted that a high proportion of patients (63.3%) who receive a diagnosis of COPD during hospital admission might not have confirmatory spirometry, suggesting this issue is more widespread in the Australian system.28 Although there has been an increase in the Medicare rebate for spirometry following the publication of all the studies included in the present review, this alone is unlikely to be sufficient to overcome all the described barriers. Despite the planning and implementation of many education programs regarding spirometry, the thoughtful and comprehensive programs instituted in some of the intervention studies appear to have resulted in less-than-optimal spirometry performance and interpretation.15,17,18
Comparison to other literature
A recent publication has analysed the barriers and facilitators to spirometry and mapped these to the theoretical domains framework.29 Despite differences in methodology, the findings of that study are strikingly similar to those of the present study; barriers around a lack of perceived utility of spirometry in diagnosing respiratory disease, issues related to skills to interpret spirometry and difficulties regarding workload and reimbursement.29 Similar to Yamada et al29 we found that GPs reported a belief that spirometry was of limited clinical utility.
Individual studies from other countries suggest some similarities and some differences with respect to barriers to spirometry compared with our findings. A nationwide survey of Canadian family practices (available as an abstract only) suggests that Canadian GPs report interest in using spirometry, but face difficulties in accessing, performing and interpreting spirometry.30 A US study reported that barriers to spirometry in primary care included time and a lack of staff,6 similar to our findings. A Dutch survey-based study concluded that lack of expertise in spirometry was ‘the limiting factor for its routine application in general practice’,7 but it is unclear whether the attitudes of GPs were explored in that study. The differences noted across countries highlights the importance of undertaking a systematic review of the literature from a single country, as in this review.
Implications for policy makers and clinicians
Overall, the findings of the present study highlight two essential problems with spirometry in primary care: the ‘why’ (ie that GPs see little reason to recommend spirometry given perceptions of limited clinical utility) and the ‘how’ (ie accessing spirometry that is accurate, convenient and available in a clinically useful timeframe). Improvements will not occur unless both these elements are addressed.
Solutions for the ‘why’ will require educational interventions, perhaps built around the documented rates of over- and underdiagnosis, and harnessing clinicians’ responsibility to minimise consequent related harms (ie incorrect diagnosis and the consequent prescription of medications that will not help, that will cause side effects and will cost both patients and government; the lack of a correct diagnosis). Shared-care consultations, where GPs see patients alongside respiratory physicians, which have been successful in diabetes,31 might have a role to play in developing momentum for this cultural change in primary care. Ongoing modelling and mentorship from clinicians with expertise in spirometry might foster spirometry use, as suggested by other research,32,33 but appropriate Medicare items would need to be developed to support such an innovation. Policy makers might need to consider other levers, such as the introduction of new Medicare items for lung health assessment, the enhancement of referral pathways for respiratory assessment or developing evidence-based strategies for enhancing care as set out in a recent report from the Lung Foundation of Australia.34
The question of ‘how’ is equally thorny. The development of new models to provide spirometry is likely required. Repeated studies cited in this review and by Yamada et al29 find that training GPs, practice nurses and/or physiotherapists does not result in accurate, correctly interpreted spirometry. In Australia, respiratory scientists are the group of allied health professionals for whom completion of spirometry is a core competency.35 Developing models that allow GPs to refer patients for spirometry undertaken by respiratory scientists, under the supervision of a respiratory physician, is a potential solution. Similar models exist for GPs accessing other clinical tests (eg echocardiograms or 24-hour electrocardiogram [ECG] monitoring that require input from cardiac sonographers or technologists and cardiologists). In addition, computerised support or external assistance with reporting might enhance GPs spirometry reporting skills.17
The level of reimbursement will be central to the viability of new models of spirometry provision. The Medicare rebate for spirometry undertaken by GPs was increased from $17.50 to $37 in 2018,36 after the publication of all studies included in this review; the impact of this increase remains to be seen. The Medicare rebate for spirometry undertaken by a respiratory scientist, under the supervision of a respiratory physician, is well below that of, for example, 24-hour ECG reporting (which requires the input of cardiac technologists and cardiologists), despite the comparable complexity involved in undertaking the studies, the costs of consumables and reporting time (T Smith and A Gillard, pers. comm., 2022). However, solutions that are appropriate for the urban setting are unlikely to meet the needs of rural and remote Australia, suggesting a broad policy discussion is required.
The Australian healthcare system is a hybrid model where the Australian Government provides universal access for basic coverage (Medicare), but individuals can supplement this with out-of-pocket payments or private insurance (although private insurance is generally not applicable to primary care or to spirometry-related costs).13 Most primary care is delivered by a fee-for-service model at little or no cost to patients.13 Reimbursement levels might influence GPs’ willingness to undertake a time-consuming test like spirometry.
Strengths and weaknesses
A key strength of this work is the inclusion of both primary care (TU) and respiratory (TS) clinicians in the research team, promoting a whole-of-system perspective. In addition, a professional medical librarian helped develop the literature search strategy, ensuring a comprehensive approach. In reviewing the literature, the authors developed categories for the barriers from the literature using an adaptive, iterative approach, similar to qualitative methodology. Further, our literature search revealed a larger number of Australian references than a recent systematic review that reviewed international findings over a similar timeframe,29 suggesting an exhaustive search process.
All studies that use inductive coding might be subject to bias, and although categories like ‘time’ and ‘cost’ are straightforward, others (eg ‘limited clinical utility’) are more complex and might have contingent relationships with other barriers. The inclusion of only Australian studies is both a strength and a weakness of this review; this decision allows a focus to a single system, allowing insights that might drive research and change within this system. However, idiosyncrasies of the Australian medical system might limit wider applicability of the findings. Of note, several of the included studies appear to come from a relatively small pool of authors, hence might reflect a constrained range of perspectives. In addition, many of the qualitative studies included data from more than 10 years ago. It is possible that the attitudes to spirometry have changed over this time. The pressures on primary care have only increased in recent years and Medicare rebates have failed to keep pace with inflation, suggesting that anything that adds to the burden in primary care, without giving a clear benefit in the view of both GPs and patients, is unlikely to have improved.
Implications for future research
An assessment of the effect of the increase in the spirometry rebate in the Australian fee-for-service model is needed. Of key importance is the need to better characterise the barrier we have categorised as ‘limited clinical utility’, with a focus on the means to address this. In addition, reviewing the available literature for facilitators of spirometry in primary care might reveal opportunities for improvements. Research on the effectiveness of respiratory scientists providing spirometry in primary care is a clear implication from this review.
Conclusions
The underutilisation of spirometry in the primary care setting continues to be problematic, despite many years of efforts on this front. Time and cost issues continue to be large barriers that might improve with the increase in the Medicare rebate. However, this study brings to light the prevalence of a perception of the limited clinical utility of spirometry for some GPs and its significance in the underutilisation of spirometry. Therefore, a multipronged collaborative approach that allows GPs to access spirometry and spirometry expertise is needed to address this critical issue of patient care.