Grants represent the lifeblood of one’s research activities, unlocking opportunities to delve into an area and make new discoveries.1 This might be facilitated by locating funding opportunities through clearing houses (eg GrantConnect and the Australasian Association for Academic Primary Care). Yet even with an opportunity identified, securing a grant can be a daunting task.
Aim
In this article, we provide guidance for novice researchers around the grant application process. We aim this article particularly, but not exclusively, towards clinicians interested in research given the current shortage of clinician researchers.2,3 Our hope is that this article will help build clinicians’ confidence to commence research, as optimal clinical and research outcomes arise when clinicians both implement and contribute to that research.4 We open with three overarching principles for approaching any research proposal, then provide specific guidance for preparing a grant application (Figure 1).
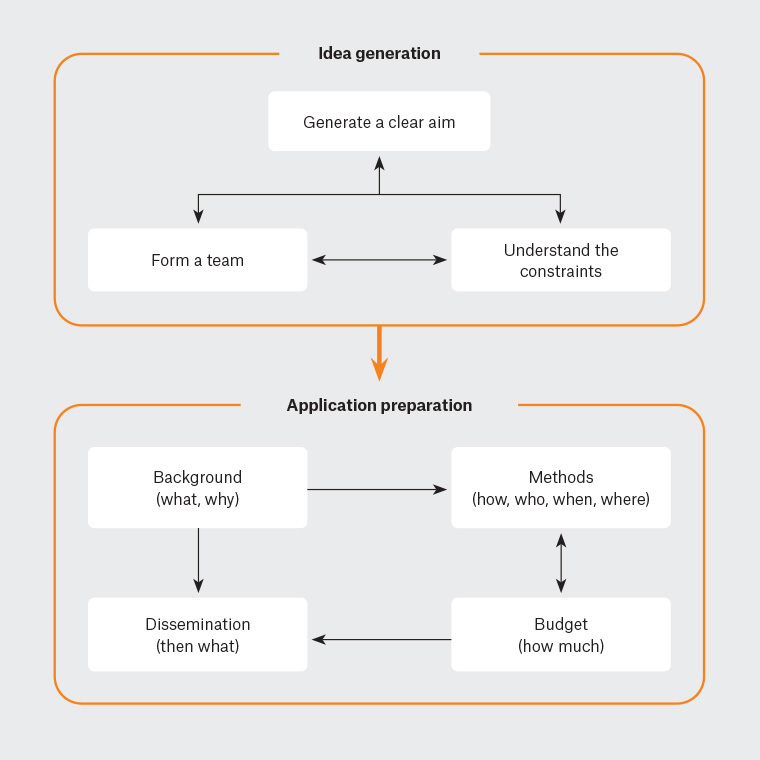
Figure 1. Preparing a clear grant application.
Overarching principles for preparing research proposals
When writing a research proposal, keep three elements in mind:
- Narration.
- Teamwork.
- Constraints.
These elements represent your overarching principles.
First, your job is that of a narrator, crafting a story that guides the reviewer to understand your central message. Key to this is creating coherence between the what, why, how, who, when and where.
Second, research requires teamwork. Particularly for novice researchers, it entails a host of unforeseen issues, including recruiting the right participants, crafting survey questions and choosing an appropriate analytic approach. Why not surround yourself with people who know the terrain and can illuminate the pitfalls?
When starting your proposal, enlist both research and industry experts. Experienced researchers can provide subject expertise and pragmatic guidance for conducting research. You might need multiple researchers to provide both sets of skills. Industry or organisational experts (eg clinicians, educators and administrators) will help you to craft a pragmatic, meaningful research question, understand participants’ needs and capacity, and translate your findings into practice. They will have the knowledge needed to boost the project’s feasibility and utility. We will expand on building your team later.
The third principle is to understand the constraints you are working within. Grants vary in scope, which dictates what you can achieve in your proposed project. For instance, a six-month $30,000 grant might allow for a brief point-in-time survey, whereas a three-year $1 million grant could enable a multi-arm, longitudinal, mixed-methods trial. Reviewers will adjust their expectations of your proposal based on the grant’s resourcing.
The application
Most grant applications have two main sections: the background and the methods. We will discuss each in turn, along with dissemination and budgeting. Throughout this discussion, we will view the application as a narrative.
The background: What and why?
In the background, your job is to guide the reader to understand why you want to answer this research question now. This means you need a literature review that will convince the reader both that a gap exists and that it is important. Accordingly, the literature review should not be exhaustive, but include only important evidence and the themes necessary for your argument.
The literature review represents an opportunity to demonstrate your project’s feasibility. This could include highlighting your research skills by referencing relevant research involving yourself or your co-investigators. This is particularly valuable if your research question stems from a previous project because it reinforces the need for your new project. Alternatively, referencing a pilot project demonstrates the project’s soundness and can guide your research design (eg informing sample size estimations, formulating survey or interview questions). Such examples will illustrate that you can complete the project.
The background section culminates with the research question. If done well, the literature review will lead naturally to this question. Craft your research question carefully because it outlines your project’s parameters. The two essential elements to include are your population and outcome(s) of interest. Table 1 provides some examples.
| Table 1. Example research questions and aims |
| Research question (funding source) |
Research aims |
What is burnout in GP registrars?5–8
(PhD project with financial support from industry organisation) |
- To understand how registrars and stakeholders conceptualise burnout and wellbeing
- To identify the risk and protective factors for registrar burnout
- To describe interventions to prevent and manage registrar burnout
|
What is the prognosis of TIAMS and TIAMS-mimics in primary care settings9
(NHMRC-funded project) |
- To establish rates of stroke, TIA, myocardial infarction and death post index event for TIAMS and TIAMS-mimic groups recruited from a primary care population
- To compare recurrence rates of stroke and TIA between these groups
|
How do older person health assessments integrate into general practice?10–13
(Student and GP registrar academic post projects funded by universities and the AGPT Program) |
- To examine what new health information is identified at a 75+ health assessment compared to the patient’s standard GP consultations from the prior 24 months
- To examine the demographic factors associated with uptake of older person health assessment over the past decade
- To explore the attitudes of general practitioners and practice nurses towards older person health assessments
- To explore the perspectives of older Australians undertaking health assessments and the perspectives of their administering clinician
|
| AGPT, Australian General Practice Training; GP, general practitioner; NHMRC, National Health and Medical Research Council; TIA, transient ischaemic attack; TIAMS, transient ischaemic attack/minor stroke. |
The methods: How, who, when and where?
The methods section will dominate your application. This is where you describe how you will answer your research question. The first step is translating your research question into more concrete research aims (see Table 1). Alongside these aims, you will likely need to prepare project objectives outlining what you will do. Setting SMART (specific, measurable, achievable, relevant, time-bound) objectives can help you track your progress.14
Once your research aims are set, you can design the study. This is a vast topic and beyond the scope of this article. Fundamentally, your task is devising a way of collecting and analysing data to address your research aims. Your research question might inform this, such as whether you pursue a quantitative or qualitative design, or a combination of the two,15 and whether you collect data at one or multiple time points (cross-sectional or longitudinal research, respectively). Table 2 provides some examples.16–18 Further information for quantitative research is available through the Oxford University Centre for Evidence-Based Medicine.19
| Table 2. Research questions and their corresponding research designs |
| Question type |
Research approachA |
Example question |
Example study design |
| How or why |
Qualitative research (various methodologies, including interviews, focus groups, document analysis) |
What are the experiences of GPs in their evaluation and management of TIA? |
Individual semistructured interview study16 |
| Who, what, where, when |
Quantitative research (various methodologies; see also cross-sectional and longitudinal research, below) |
How do GP patients anticipate they would respond to TIA symptoms? |
Cross-sectional, questionnaire-based study incorporating clinical vignettes17 |
| Frequency/prevalence |
Cross-sectional research |
What is the prevalence and what are the associations of new TIA presentations to GP registrars? |
Cross-sectional analysis of data collected during GP consultations18 |
| Changes over time or prognosis |
Longitudinal research |
What is the prognosis of TIAMS in community practice? |
Inception cohort study9 |
ANot mutually exclusive.
GP, general practitioner; TIA, transient ischaemic attack; TIAMS, transient ischaemic attack/minor stroke. |
The details you need to include in your application will depend on the grant’s requirements and your study design. However, there are three basic factors you will always need to describe:
- Participants: Who will you recruit? When? Where from? How will you recruit them?
- Data: What will you collect (eg survey responses and audio recordings)? How will you collect this?
- Analysis: How will you derive meaning from the data?
Ethical considerations will be thoroughly explored when preparing your ethics application. Nonetheless, it is important to contemplate them at the application stage; research without an articulated ethical approach is unlikely to be funded. In the application, consider matters such as whether pre-existing relationships between researchers and participants could be viewed as coercive or whether you will compensate individuals for participating. These matters have logistical and financial implications that need to be addressed. The National Statement on Ethical Conduct in Human Research provides further guidance on these points.20
So far, we have covered the what, why, how, when and where. We have touched on the who (ie research participants), but the ‘who’ also has another component – who will do what in the project? You need to map the capacity and skills of team members against the project tasks. This list is long, including preparing ethics applications, recruiting participants, collecting data, analysing data, writing reports, managing timelines and budgets, and organising meetings. You might need additional people to fill skill and capacity gaps. Table 3 provides some examples. Depending on the grant’s scope, you might distinguish between your research team and a steering committee. Research team members are responsible for completing the project and so will be the most heavily involved. Steering committee members can be called upon at different points to provide higher-level direction. Beyond this, you might also have others whose expertise you draw upon as needed. When forming both your research team and your steering committee, choose people whose strengths complement each other. A multidisciplinary team means each person brings a different perspective, enriching the research approach and the interpretation of findings. You might produce a long list of team members, with collaborators’ involvement varying. In developing your list, remember the constraints of your grant and explore work-arounds, such as in-kind support.
| Table 3. Overview of roles for research teams |
| Role |
Functions |
Typical level of involvement |
| Academic consultant |
Providing subject matter and/or methodological advice, supporting interpretation of data |
Steering committee |
| Principal investigator (also referred to as chief investigator) |
Making decisions about the project, leading the project, mentoring other team members |
Research team |
| Organisational representative |
Providing guidance about systems and policies, advocating for your research to others in the field |
Steering committee |
| Participant representative |
Providing the perspective of the participant group (eg patients) to maximise acceptability of the research |
Steering committee |
| Project manager |
Monitoring and revising timelines, managing reporting requirements, overseeing project budget, organising meetings, coordinating team members |
Research team |
| Research assistant |
Supporting development of ethics applications, collecting data, cleaning data, analysing data, preparing reports |
Research team |
| Statistician |
Providing advice for study design (particularly data collection methods), analysing quantitative data |
As needed |
Dissemination: Then what?
The funder will likely be interested in the project’s potential impacts to get maximum value from their investment. This means you should consider your plans for the project’s findings and its impact after completion. Traditional dissemination strategies, such as conference presentations, media releases and academic publications, can help prevent unnecessary duplication of research and guide future advances. Less traditional research outputs (eg webinars, podcasts, infographics and videos) can target non-academic audiences, particularly those who can implement or benefit from your findings (ie research translation). Also consider who you can partner with to translate the findings. Including organisational representatives within the project’s steering committee from the start can build their investment in the project, encouraging them to help translate the project’s findings.
The budget: How much?
Given that your motivation for writing the application is to acquire funding, spend plenty of time preparing the budget. The largest budget items tend to be salaries, which require you to forecast the time each team member will need to fulfil their duties. Also remember those individuals beyond the immediate research team (eg academic consultants, statisticians and steering committee members). You might need services and material resources, such as transcription, survey or software licences, or participant reimbursements. Experienced researchers and project managers can help identify costs and provide realistic estimates of time requirements for different phases. If you are providing an itemised budget, also ensure your application justifies each expense. To supplement budget constraints, again consider opportunities to reduce costs through in-kind contributions, particularly from project partners. Further guidance regarding budgeting is readily available.21
Conclusion
Grant writing is nuanced and can be daunting. Viewing your task as that of a narrator can simplify the process to answering basic questions: What is the topic? Why is it important? How will you examine it? Who will work with you? When and where will you do the research? Then what will you do? How much will it cost? Throughout this process, create a diverse, complementary team to support you, draw on their unique strengths and be mindful of the constraints you are working within. We conclude with general tips (Box 1) for preparing a grant application to maximise the chances that your next application will be successful. Best of luck!
| Box 1. Tips for preparing a grant application |
- Read the guidelines:
- Ensure you and your team are eligible to apply for the grant.
- Understand what you are and are not permitted to spend your budget on (eg salaries, conference attendance, software, on-costs).
- Know the word limits for different sections – it is frustrating to write a 5000-word literature review and find you only have 750 words for it in the application.
- Review the selection guidelines to understand what the reviewers are looking for and how they will be assessing applications.
- If in doubt about any part of the application process, ask the funders for clarification.
- Make it easy for the reviewer:
- Ensure your overarching message is consistent across the application.
- Grants often span multiple sections; do not be afraid to repeat key points across sections.
- Use short sentences.
- Avoid jargon and abbreviations where possible, and clearly define terms. Unless otherwise stated, assume the reviewers have limited background in your topic.
- Be generous in your timelines:
- Allow plenty of time to plan the project – if your application is rushed, it will show.
- Allow at least three months to obtain ethics approval, particularly if it needs to go to the Human Research Ethics Committee full panel.
- Optimise your timelines so that there is always something for people to do. For example, what will your research team do while waiting for ethics approval? Could you apply for ethics before the grant period commences?
- Recruitment can take a long time, so factor in a margin for overcoming hurdles.
- Do not reinvent the wheel:
- If there are validated surveys or procedures, use them (note: these might require permission and/or payment).
- Look at previous successful applicants’ projects to understand what the reviewers are looking for.
- If you will be analysing statistics, consult a statistician during project design to ensure that the data you collect will let you answer your research questions and that you can expect sufficient statistical power to answer your questions. You can also seek quotes for outsourcing statistical analysis to ensure rigour.
|
Key points
- A good application is like a story, guiding the reviewer to your central message.
- Answer key questions of what, why, how, who, when, where, then what and how much.
- Research requires teamwork, so leverage others’ expertise.
- Be mindful of the resource, financial and time constraints set by the grant.
- Understand the guidelines for the grant to maximise the suitability of your proposal.