Case
A woman, aged 75 years, presented with recurrent lower limb ulcers spanning three decades and bilateral lower limb swelling (Figure 1). Past medical history included deep vein thrombosis (DVT), type 2 diabetes, hypothyroidism, obesity, chronic back pain, osteoarthritis and depression. The woman was homebound and socially isolated but mobilised with a four-wheeled walker. The patient was admitted to hospital for management following unsuccessful treatment in the community with home nurse visits under general practitioner supervision.
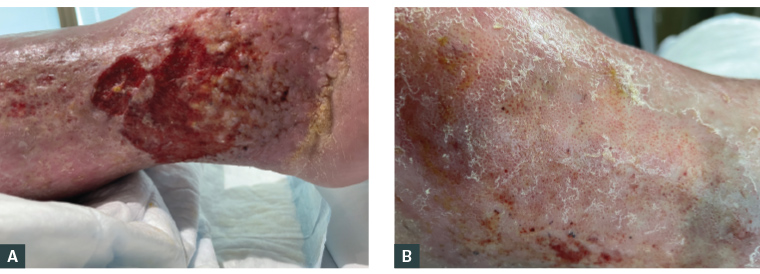
Figure 1. Legs at presentation. A. Ulcer over the right lateral malleolus demonstrating shallow erosion with irregular borders, surrounding haemosiderin deposition and lipodermatosclerosis. B. Left lateral malleolus venous dermatitis of the skin with atrophie blanche-like changes (depressed white areas with prominent red dots of dilated capillaries, often associated with moderate–severe pain).1
Question 1
What is the primary diagnosis?
Question 2
What are the common causes and features of lower limb ulcers?
Answer 1
The primary diagnosis is venous ulceration, indicated by characteristic features shown in Figure 1. The patient carries risk factors for venous insufficiency including obesity, immobility and a history of DVT.
Answer 2
Common aetiologies of lower limb ulcers are venous, arterial and neuropathic (Table 1).2 They affect 1–1.5% of the population and have an estimated yearly economic burden greater than $1 billion in Australia.2,3 Chronic ulcers (more than three months) persist despite treatment, remaining in an inflammatory state driven by the presence of a biofilm.2,4
| Table 1. Common aetiologies of leg ulcers |
| Aetiology |
Pathophysiology |
Risk factors |
Diagnosis |
Characteristic features |
| Venous |
Venous or lymphatic blockage or insufficiency |
Varicose veins, DVT, reduced mobility |
Clinical; ultrasound confirms venous incompetence or thrombus |
- Shallow irregular ulcer with granulating or fibrous bed in gaiter region
- Significant oedema and exudate, lipodermatosclerosis, haemosiderin staining, atrophie blanche
- Variably painful5
|
| Arterial |
Reduced blood flow resulting in ischaemia |
Age, diabetes, smoking, hypertension, hyperlipidaemia, coronary and cerebral atheroma |
Clinical; ankle–brachial PI accords with the severity of ischaemia |
- Well-demarcated ulcers with poorly granulating wound bed and necrotic edge
- Occurs distally and over bony prominences
- Associated hair loss, shiny skin, absent peripheral pulse, slow capillary refill
- Severe pain, worse with elevation5
|
| Neuropathic |
Loss of sensation or reflex resulting in unrecognised traumatic or pressure ulcer formation |
Diabetes, alcohol abuse, nutritional deficiency, spinal cord dysfunction, autoimmune disorders |
Sensation testing |
- Punched-out, calloused edge located on pressure points
- ‘Charcot’ deformity, decreased sweating with dry, scaly appearance
- Painless, often deep progression and sepsis5
|
| DVT, deep vein thrombosis; PI, pressure index. |
Case continued
Doppler ultrasound showed no evidence of new DVT and, clinically, the patient was not in heart failure. C-Reactive protein was 26 mg/L (mildly elevated), with other inflammatory markers within normal limits. In the absence of signs of infection, wound swab and antibiotics were not indicated because chronic wounds have bacterial colonisation and biofilm.5
All ankle pulses were palpable, excluding significant large artery ischaemia. Despite the patient’s poor diet, iron and vitamin B12 deficiencies and protein–calorie malnutrition were excluded.
Question 3
What contributing factors to non-healing should be considered?
Question 4
What is your management for this patient?
Question 5
What further investigations would you consider in the event of treatment failure?
Answer 3
Potential reasons for delayed wound healing include:
- failure to address reversible comorbid factors
- the presence of irreversible risk factors
- inappropriate wound management.
Reversible factors provide targets for intervention and include obesity, smoking, malnutrition, immobility, infection, wound biofilm, oedema, vascular insufficiency and medications (eg steroids or non-steroidal anti-inflammatory drugs).6,7
Non-modifiable risk factors include age (>55 years) and medical history.6,7 Here, the patient’s age, chronic back pain and life-long obesity are non-modifiable risk factors.2,6 Further, her venous insufficiency and immobility are refractory to treatment.
Wound care was complicated by social isolation. Obesity and chronic pain prevented self-managed dressings, rendering the patient reliant on in‑home nursing, which inherently carries inconsistencies in availability and wound care experience.
Answer 4
Successful management of chronic wounds requires recognition of the financial and social impacts to the patient. Although the cost of wound care can be a barrier to treatment, chronic ulcers that are not pressure related, ischaemic or malignant may be addressed at low expense with three management principles: reduce oedema, manage biofilm and prevent exudative dermatitis (Table 2).3,8 The role of invasive treatments for venous ulcers, including varicose vein surgery, as adjuncts to compression remains controversial. These treatments should be avoided in the initial phases of management.9,10
| Table 2. Basic concepts of wound management |
| Disrupt the biofilm |
- Chronic wounds are arrested in the inflammatory phase due to the presence of biofilm
- Debride the wound bed at each dressing change
- A debriding dressing is a less efficient alternative to mechanical debridement
|
| Protect against exudate |
- The wound edge skin is the source of new epithelium that will migrate across the wound bed
- Wounds cannot produce oils necessary for protection, so must be protected against exudate
- Remove hyperkeratosis that traps exudate on the skin
- Apply barrier cream to the wound and surrounding skin
- Use a superabsorbent dressing to prevent exudate retention on the skin
- Change dressings before they become saturated
|
| Reduce oedema |
- Oedema results from gravity, exacerbated by loss of calf muscle pump (immobility), organ failure or venous and lymphatic incompetence/obstruction
- Reducing oedema reduces cellulitis
- Gravity-related oedema needs antigravity measures (eg compression garments, bed rest with lower limb elevation)
|
Answer 5
Leg ulcers can have uncommon causes. Should first-line strategies fail, further investigations might aid in the diagnosis and management of uncommon aetiologies (Table 3). Possibilities include atypical infections (Mycobacterium), vasculitis and pyoderma gangrenosum, malignant transformation (Marjolin ulceration) and dermatitis artefacta (self-harm). What is ordered is dependent on multiple factors, including history, risk factors, comorbidities, medications and clinical suspicion.
| Table 3. Investigations for a leg ulcer work-up |
| Investigation |
Indication |
Examples |
| Baseline blood tests |
To rule out underlying systemic conditions such as diabetes, chronic kidney disease, liver disease |
- Full blood count
- ESR/CRP
- HbA1c
- BSL
- Lipid profile
- eGFR/creatinine
- LFTs
|
| Nutritional screening |
To assess nutritional status and/or deficiencies |
- Serum protein
- Albumin
- Vitamin B12/folate
- Zinc
- Iron studies
|
| Vasculitis screen |
To rule out underlying vasculitic process |
- ANA/RF/ANCA
- Complement C4
- Paraproteins
- Circulating immunocomplexes
- Urine analysis (proteinuria/haematuria)
- Immunohistopathology of skin biopsies
|
| Thrombophilia screen |
To exclude clotting disorders |
- PT/aPTT
- Factor V Leiden mutation
- Factors VIII and IX
- Factor 2 (prothrombin) mutation
- Antithrombin III
- Proteins C and S
- Lupus anticoagulant
- Fibrinogen
|
| Tissue biopsy |
Perform when diagnosis unclear, suspected malignancy, atypical infection or if wound not healing despite appropriate treatment |
- Wedge biopsy (preferred)
- Punch biopsy
- Tests ordered include histopathology and culture for atypical infection
|
| Microscopy, culture, sensitivity |
Perform only in the presence of signs of infection |
|
| Imaging |
Exclude underlying osteomyelitis, foreign body or malignancy |
|
| Vascular imaging |
To assess for venous reflux and obstruction and to gather further anatomical information for treatment and planning |
- Duplex ultrasound
- CTA
- MRA
|
| ANA, antinuclear antibodies; ANCA, antineutrophil cytoplasmic antibodies; aPTT, activated partial thromboplastin time; BSL, blood sugar level; CRP, C-reactive protein; CT, computed tomography; CTA, computed tomography angiography; eGFR, estimated glomerular filtration rate; ESR, erythrocyte sedimentation rate; HbA1c, glycated haemoglobin; LFTs, liver function tests; MRA, magnetic resonance angiography; MRI, magnetic resonance imaging; PT, prothrombin time; RF, rheumatoid factor. |
Case continued
The wounds were managed using the three first-line principles:
- Disrupt biofilm
- Daily wash under running water with mild decontaminant (eg Prontosan [B Braun Medical AG] or povidone iodine) and debriding agitation with cloth
- Mechanical debriding dressing (eg Mesalt [Mölnlycke Health Care]) cut to fit the wound bed
- Prevent exudative dermatitis
- Barrier cream (eg Cavilon [3M Cavilon Skin Care Solutions] and Sudocrem [Teva Pharma]) applied to the wound and surrounding skin, together with a low-potency steroid (betamethasone valerate 0.02%) to reduce the symptoms of dermatitis
- Superabsorbent dressing changed daily
- Cotton sleeve for dressing retention (avoid tapes)
- Reduce (gravity-related) oedema
- Graduated compression or short-stretch inelastic bandaging or layered tubular bandage
- Bed rest with lower limb elevation to the level of the heart
The management regime resulted in reduced pain and visible wound improvement (Figure 2A–C). The patient was discharged to community care and fitted with Class 3 compression garments after 18 days. At the four-month follow-up, the wound demonstrated sustained healing (Figure 2D), with significant improvement in the patient’s quality of life.
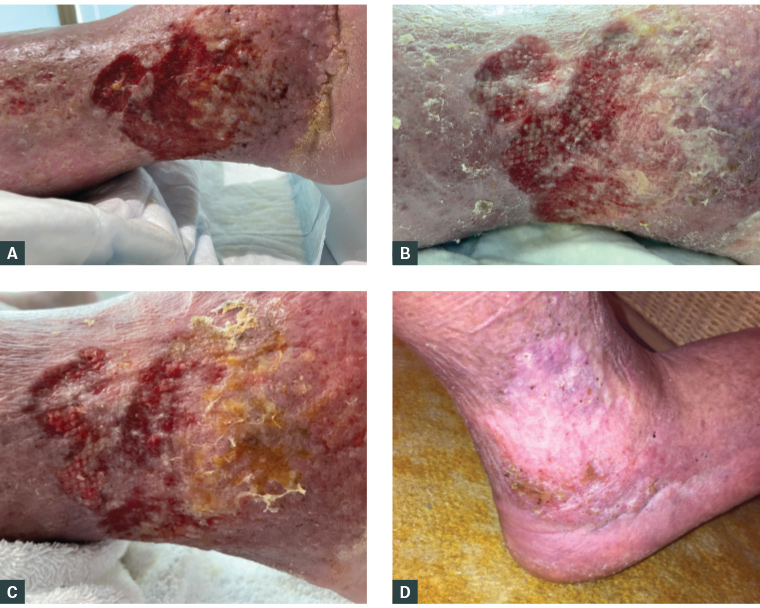
Figure 2. Right leg wounds following initiation of first-line principles. A. Day of admission. B. Day 12. C. Day 17. D. At the four-month review after discharge.
Key points
- Skin protection, biofilm disruption and control of oedema through graduated compression and avoiding dependency are minimum requirements for the healing of chronic wounds where these factors are at play.
- Addressing these management principles is not costly.
- Acknowledging the psychosocial impact of chronic wounds is important to engage the patient.