Most inflammatory and neoplastic skin disease is managed by general practitioners (GPs), comprising around 15% of consultations.1
Punch, shave and incisional biopsy, curettage, formal excision with closure and shave excision are essential in diagnosing and treating skin conditions.2 Which is used depends on multiple factors, including the presenting condition, patient and clinician variables and clinician intent. Surgery might be diagnostic, curative or both. Procedure choice must balance the potentially conflicting demands of cure, convenience, cost and cosmesis.
Types of shaves
Shave procedures require good lesion selection and technique. Needing minimal equipment, they are quick, producing good cosmetic and functional outcomes if performed well.3 Doctor-related benefits include no need for advanced surgical facilities, nursing assistance, suture placement or removal, thus minimising costs. The simplicity and brevity of a shave allow single or multiple procedures to be performed immediately after lesions are identified. Postoperatively, there is minimal restriction of patient activity.
Shaves fall into three categories:
- shave biopsy where the intention is sampling only
- shave excision where the intent is to remove a lesion in width and depth
- shave procedures as part of curettage and cautery.
Confusion exists where shave excision and biopsy are conflated. Studies fail to consider clinician intent, expertise and training when reporting outcomes.4,5
A shave can harvest specimens that range in width from 2 mm to many centimetres. Depth can range from capturing epidermal elements only to superficial, mid- or deep dermis and even subcutis.2 The width and depth of a shave are tailored to lesion size and clinician intent.6
Where the diagnostic histopathology is confined to the epidermis or superficial dermis, a shave biopsy producing a thin and flat specimen of epidermis and upper dermis (<1 mm) is all that will be needed (see Figure 1 for depiction of sampling depth).7 Examples include warts, skin tags, superficial basal cell carcinoma (BCC) or squamous cell carcinoma (SCC) and seborrhoeic or actinic keratoses.8
Shave excisions yield a thicker disc of tissue extending to at least the mid-dermis (1–4 mm deep; see Figure 1).7 This provides a diagnostic specimen that has removed the lesion in width and depth.9
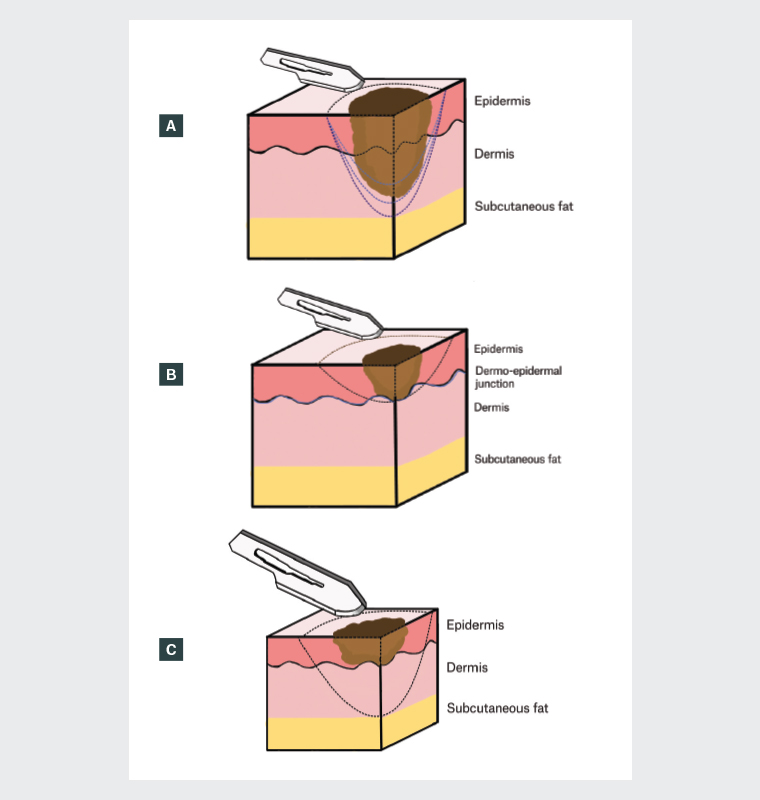
Figure 1. Diagrams of (A) shave biopsy with varying depths, including partial sampling with no intention to completely remove (light blue dotted line), shave prior to immediate curettage and cautery (medium blue dotted line) and shave excision of a lesion where melanoma is a differential diagnosis and the lesion is in situ or thin, down to mid-reticular dermis or at the junction with subcutis (dark blue dotted line); (B) superficial shave depth; and (C) shave excision depth, all in relation to the lesion.
Indications
Shaves are primarily used in the diagnosis and management of benign and malignant skin neoplasia but have a role in inflammatory dermatoses.10
Inflammatory skin disease
A common issue is distinguishing an intraepidermal carcinoma (IEC) from background psoriasis or eczema.11 A small shave sampling to the superficial dermis will differentiate. In cutaneous T-cell lymphoma, the presence of epidermotropism (malignant T cells infiltrating the epidermis in the absence of spongiosis) is an important diagnostic feature.12 Shaves provide more comprehensive sampling of possibly involved epidermis.
Diagnostic features of autoimmune blistering diseases are essentially confined to the epidermis and superficial dermis.13 The ideal specimen in conditions such as bullous pemphigoid, linear IgA disease or pemphigus vulgaris is a new, intact blister, which is readily harvested by shaving out a small intact blister to the mid-dermis with a margin of adjacent skin. The specimen is transected to separate the intact blister from the perilesional skin. The former is submitted for histopathology and the latter for direct immunofluorescence.13
Seborrhoeic keratoses
Common benign verrucous lesions are treated for reasons of cosmesis, irritation and diagnostic uncertainty. Malignancies complicating or mimicking seborrhoeic keratoses include IEC, SCC and BCC and melanoma.14,15 Lesion inflammation can be symptomatic and might indicate associated malignancy.16 Management options include cryotherapy, chemical peels, dermabrasion, CO2 laser, curettage and even formal excision.17 If clinically consistent with seborrhoeic keratosis, a superficial shave of the entire lesion to the superficial dermis will ‘cure’ the lesion and confirm the diagnosis. If clinical diagnostic doubt exists, a deeper shave or formal excision depending on circumstances is needed.
Warts
A simple shave, possibly with light electrocautery, can be a useful modality for viral warts, especially if hyperkeratotic, treatment resistant or diagnostically challenging. IEC, SCC and even melanoma can mimic warts. With any wart treatment, recurrence is not uncommon.18
Benign naevi
Shaves are used to remove domed dermal naevi. This can leave residual naevus cells and be complicated by recurrence,2 often manifesting as irregular pigmentation confined to the scar. Extension beyond the scar raises the possibility of melanoma.
Solar keratosis
Solar keratoses have an increased albeit low risk of malignant transformation.19 Often hyperkeratotic, they are one of many causes of keratin horns.20 Treatment resistance is seen, and IEC or invasive SCC can be differential diagnoses. As purely an epidermal lesion, a thin shave into the papillary dermis will usually cure and remove diagnostic doubt.
Keratinocyte malignancy
Keratinocyte malignancy includes IEC, invasive SCC, BCC and keratoacanthoma. Shaves can be diagnostic to guide management, definitive treatment or part of a curette. Patients with a high burden of skin malignancy can easily have multiple shaves during the same consultation. This eliminates non-compliance and reduces time and financial burdens.
Lesions where the differential is IEC or superficial BCC require sampling to the superficial dermis at one or multiple sites depending on the lesion dimensions.8 Large BCCs can harbour more than one growth pattern. For more deeply invasive keratinocyte malignancies, a sample to the mid-dermis or deeper might be needed.
Keratoacanthomas are effectively managed with curettage.21 An initial shave excision to the deep reticular dermis followed by curettage and cautery to the resultant surgical defect avoids specimen fragmentation, making histopathological interpretation easier.22 This method can be used in small BCCs and well-differentiated SCCs23 by practitioners confident in lesion selection and technique.
Melanocytic lesions
Shave procedures in pigmented lesions is an area of controversy in the context of melanoma management. Accurate biopsy of a potential melanoma will allow for the pathological determination of tumour depth, which influences management and prognostication.7 Therefore, an incorrect biopsy technique can miss or delay the diagnosis of melanoma. Australian guidelines recommend elliptical excision as the preferred diagnostic procedure.24 The same guidelines state that ‘deep shave excision (saucerisation/scoop) and punch excision methods might also be used for complete excision but are more often associated with positive margins than elliptical excision with primary closure’.24 The relative merits of the two procedures are presented in Table 1.
| Table 1. Comparison between shave excision and formal ellipse25,26 |
| Shave excision |
Formal ellipse |
| Quick same-day procedure |
Greater time commitment |
| Requires simple surgical skills |
Requires more advanced surgical skills |
| Minimal equipment needed |
More equipment required (sutures, needle holder and forceps) |
| No need for advanced surgical facilities |
More likely to require operating theatre |
| Simple aftercare with no suture removal |
Patient has to return for removal of sutures |
| No/minimal restriction of activity postoperatively |
Activity restricted postoperatively and there is a risk of wound dehiscence |
| More likely to transect base |
Little risk of transecting base |
| Removes suspect lesion with minimal uninvolved tissue |
Greater removal of normal skin and increased risk of deep structure injury due to excision into the subcutis |
Shave excision is appropriate in carefully selected circumstances:25
- initial removal of small pigmented lesions where in situ or superficially invasive melanoma is a differential
- sampling of lesions where complete excision is untenable due to size or site
- mapping the extent of large lentigo maligna.
A major concern when it comes to shaving melanocytic lesions is the risk of transecting a melanoma,27 thus compromising the Breslow thickness needed to estimate prognosis. To avoid this, lesion selection is of utmost importance. Adequately performed shaves can successfully remove thin, small-diameter melanocytic lesions in their entirety (Figures 2,3). Conversely, lesions that extend to the deep dermis and are palpably raised on examination should not undergo shave excision.25
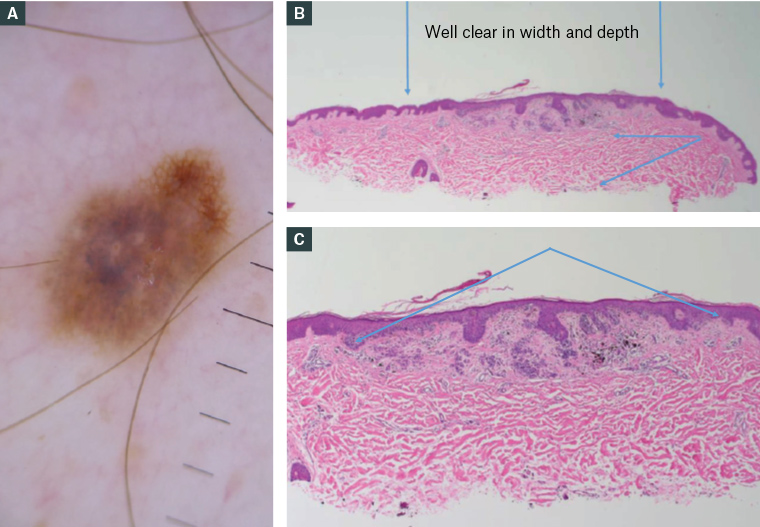
Figure 2. (A) A changing pigmented lesion on the back that underwent shave excision. (B) Histopathology slides confirming the excision is well clear in width (denoted by the vertical blue arrows) and depth (as per the horizontal/diagonal blue arrows to the right). (C) In the photomicrograph, the lesion is seen in full as symmetrical with terminal nests (area in between the blue arrows), consistent with a dysplastic naevus.

Figure 3. Photomicrographs of (A) a shave excised melanoma in situ removed from a patient’s back. Note that (B) and (C) show that the shave width (black lines) and depth are such that the entire lesion is captured.
Where melanoma is a significant differential, shave excision is only appropriate if the clinician is confident the lesion can be removed in width and depth. In the authors’ experience, and supported by the studies by Gambichler et al,9 Tran et al,25 Shao et al,28 Pitney and Muir29 and Brown et al,30 this refers to a lesion less than 10 mm in diameter that is flat without clinical or dermoscopic evidence of deep extension. The lateral extent of the shave is the same as for an ellipse, and the shave should reach the deep reticular dermis (ie within the dermis but just above the subcutis).
Larger lesions that are not amenable for complete excision (due to their location or risk of poor cosmesis) can undergo partial biopsy in the form of a shave. This is true for conditions such as lentigo maligna, where a large shave biopsy might be preferred because it provides the pathologist with a greater amount of epidermal tissue for assessment, allowing for adequate appreciation of the heterogeneity in the histopathology of lentigo maligna lesions.31
A clinician confronted by a suspect skin lesion might opt to monitor. This is not without risk of disease progression and patient non-compliance. These risks should be balanced against the benefits of immediate excision.
Contraindications for shave procedures
There are many instances where shaves are inappropriate. For specimens into the subcutis, depending on size, incisional biopsy or elliptical excision are preferred. Suitably sized punch or incisional biopsies are needed to diagnose panniculitis, vasculitis, lupus or hair follicle disorders.32 Raised lesions suspicious for melanoma should not undergo shave excision.25 Shaves become less useful as lesion diameter and depth increase.8 Any partial biopsy leaves the patient with their presenting complaint and risks missing foci of malignancy.
Shave procedure technique
The equipment needed to perform a shave procedure is listed in Table 2. Steps in the shave procedure technique are as follows:
- Accurately delineate, with a marker, the horizontal dimensions of the shave, measuring a 1- to 3-mm margin before shaving.
- Obtain a baseline image to identify the site on review. This is crucial if any further procedure is needed.
- Apply preoperative antiseptic solution.
- Infiltrate local anaesthesia, either plain or lignocaine with adrenaline. Infiltration into the dermis to a level of turgidity will assist with the ease of the procedure.
- Place the skin under three-point tension and shave out the marked area. The depth of the shave varies with lesion characteristics and clinician intent.
- Haemostasis is readily achieved with time, pressure and a calcium alginate dressing. Aluminium chloride or light electrocautery can be used if needed.
- Indicate on the pathology form your intention to fully excise or merely sample so the reporting pathologist will report margins.
Figures 4 and 5 show examples of the shave procedure technique.
| Table 2. Equipment for shave procedures |
| Equipment |
Comments |
| Digital camera |
Photograph the lesion; include dermoscopic photograph if possible |
| Local anaesthesia |
Lignocaine 1% ± adrenaline (1:100,000) |
| Syringe, drawing-up needle, injecting needle |
3-mL syringe, 18- to 21-gauge drawing-up needle, 30-gauge injecting needle |
| Skin preparation solution |
Isopropyl alcohol, chlorhexidine, povidone-iodine |
| Gauze |
|
| Surgical marking pen |
|
| Biopsy instrument |
No. 10 or No. 15 scalpel blade, biopsy blade or Stiefel curette |
| Haemostatic agents |
Aluminium chloride 20% in alcohol electrocautery |
| Dressing |
Adhesive dressing ± calcium alginate dressing |
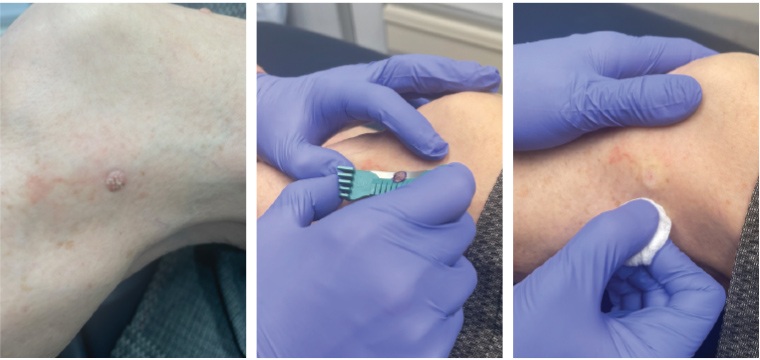
Figure 4. Shave biopsy of a seborrhoeic keratosis on the lower limb.
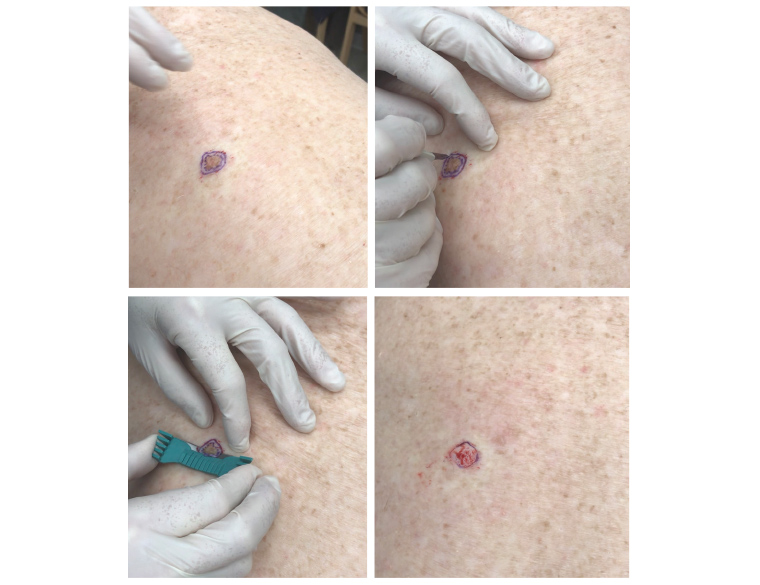
Figure 5. Shave excision of a pigmented lesion on the back, diagnosed as melanoma in situ, with clear margins on shave excision and no residual on wide local excision.
Key points
- Shave procedures performed on suitable lesions by trained practitioners are useful for sampling or definitive removal of suspect lesions.
- Shave procedures can effectively eliminate non-compliance.
- Shave procedures are cheap, same-day, quick-to-perform procedures requiring only basic surgical facilities.
- Cosmesis can be expected to be satisfactory or better.
- Where the intent is complete removal, margin involvement is rare given good lesion selection and technique.
- Shave procedures produce significant direct and indirect cost savings.