Recurrent urinary tract infections (rUTIs) are a common condition that can be debilitating for patients and pose an increasing challenge in the primary care setting. A narrative review of the available management strategies for rUTIs was conducted and we present an approach to the diagnosis and management of rUTIs. We hope to provide general practitioners (GPs) with a resource to aid initiating management through to when to escalate care to a urologist.
Definitions
Uncomplicated, or ‘simple’, urinary tract infection (UTI) refers to symptoms isolated to the bladder only.1 Recurrent UTIs (rUTIs) are defined as two or more episodes of uncomplicated UTI in the preceding six months or three or more episodes diagnosed over one year.2–6 Pregnant patients are considered to have rUTIs if they have two or more episodes of UTIs during their pregnancy.7 rUTIs can impact a patient’s quality of life, as persistent symptoms can be debilitating and is a risk factor for antimicrobial resistance and severe infections.3,4,6
The pathophysiology of rUTIs includes bacterial persistence or bacterial reinfection. New infections with the same organism refer to infection persistence, often due to inadequate initial treatment of ongoing nidus of infection. Reinfection refers to infections of the urinary tract with different bacterial organisms, the same organism after more than two weeks, or a sterile intervening culture.3,8 In practice, GPs might be required to exercise judgement as to whether urine microscopy/culture/sensitivities (MCS) returning the same organism is due to bacterial persistence or reinfection. Understanding the prevalence of the causative organisms in rUTIs can be helpful (Table 1). Common causative organisms such as Escherichia coli isolated on repeat urine MCS can be due to bacterial reinfection or bacterial persistence, whereas more resistant organisms such as Pseudomonas aeruginosa infections are more likely due to persistence.
| Table 1. Causative organisms and risk factors for recurrent urinary tract infections in female and male patients |
| Female |
Male |
| Causative organisms2,10 |
Escherichia coli (70–95%)
Klebsiella pneumonia (10%)
Staphylococcus saprophyticus (5–10%)
Enterococcus faecalis (5%)
Proteus mirabilis (4%)
Pseudomonas aeruginosa (1%) |
Escherichia coli (55%)
Klebsiella pneumonia (7%)
Enterococcus faecalis (10%)
Proteus mirabilis (9%)
Pseudomonas aeruginosa (7%) |
| Risk factors for recurrent UTIs |
Anatomical risk factors in females9,11
- Shorter urethra
- Close proximity between urethral and rectal openings
- Anterior vaginal wall prolapse or urethral diverticulum causing incomplete emptying
|
Incomplete emptying and high post void residual in males9,12
- Prostate cancer
- Benign prostatic hypertrophy
- Urethral stricture
- Urethral cancer
- Phimosis
|
Postmenopausal female6,13
- Oestrogen depletion, causing:
- Vaginal atrophy (thinning of the vaginal epithelium, reduced elasticity, thinning of vaginal rugae and reduced vaginal secretions)
- Atrophic vaginitis (inflammatory process associated with thinning of the vaginal epithelium causing dryness, itchiness, pain, dyspareunia and incontinence)
|
Reservoirs for infection in males12
|
Females in all stages of life6,14,15
- High frequency of sexual intercourse
- New or multiple sexual partners
- Spermicidal and diaphragm use
- Childhood UTI
- Maternal UTI (due to possible genetic factors or shared environment exposures)
- Patient or family history of blood group antigen non-secretor status (non-secretor females have vaginal epithelium that bind pathogens more avidly, leading to increased risk of colonisation)
|
All patients9,12,16
- Advanced age
- Functional deterioration leading to institutionalisation
- Conditions that suppress immunity (eg diabetes, chronic illness)
- Reservoir for infection
- Bladder/renal calculi
- Urothelial carcinoma of the bladder
- Bladder/calyceal diverticulum
- Constipation causing inadequate bladder emptying
- Poor perineal hygiene (pad use, diarrhoea, incontinence)
- Functional risk factors
- Dysfunctional voiding
- Pelvic floor dysfunction
- Neurological conditions (eg multiple sclerosis, Parkinson’s disease, spinal cord injury, peripheral neuropathy)
- Medications (eg SGLT inhibitors)
|
| SGLT, sodium–glucose transport protein; UTI, urinary tract infection. |
Prevalence
Urinary tract infections are more common in females, with 30–50% of females experiencing a UTI in their lifetime, as compared with men at 20%.2–4 One-quarter of women who experience UTI go on to experience rUTI.2 Given the prevalence of uncomplicated UTIs in male patients without predisposing risk factors is low (<1% in males aged 15–50 years),2 males with simple or rUTIs require further investigation and referral for urology opinion.
Aetiology and risk factors
Urinary tract infection is commonly caused by ascending infection into the bladder via the urethra by rectal bacterial flora that reside in the periurethral area that replicate in the bladder to cause cystitis and urinary symptoms.9 Other causes include colovesical fistula, urethral instrumentation and systemic seeding.9 Uropathogens causing recurrent rUTIs are similar to those causing acute UTI (Figure 1).2
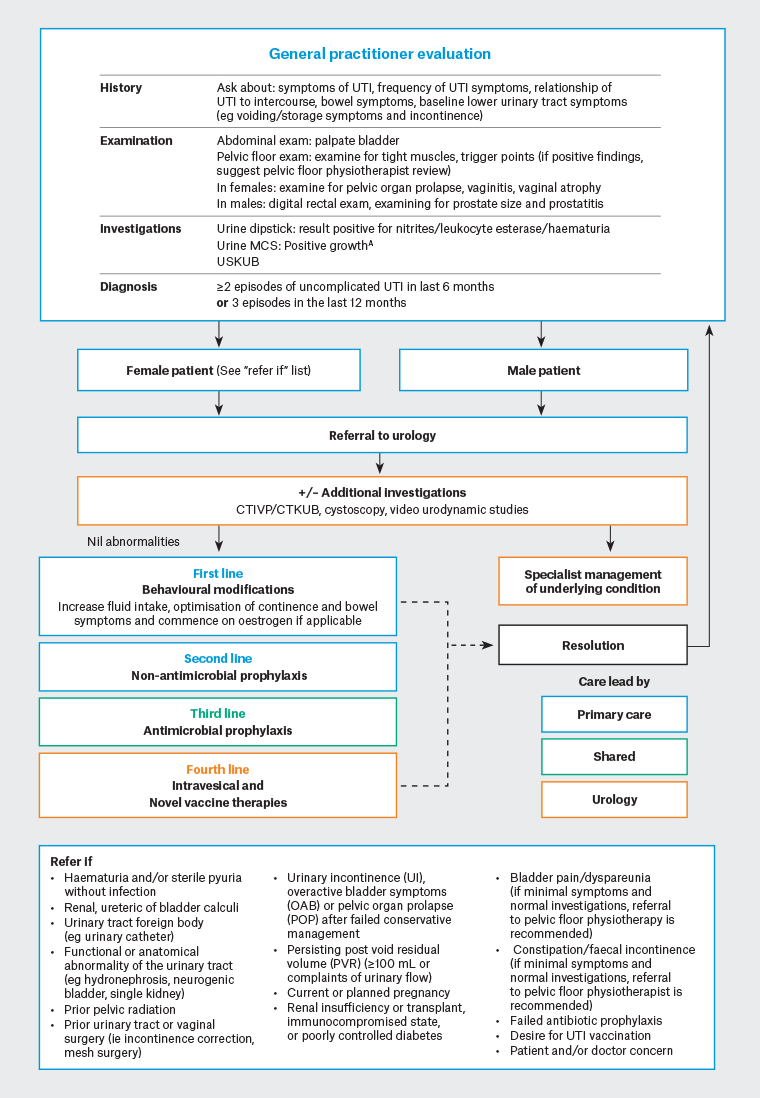
Figure 1. Diagnostic and management algorithm for recurrent urinary tract infections. Click here to enlarge
ANegative urine MCS does not exclude UTIs in the presence of symptoms,17 asymptomatic bacteriuria should not routinely be screened for and often require no treatment. Circumstances for the treatment of asymptomatic bacteriuria include pregnant patients or prior to a urological procedure, which might risk breaching the urinary mucosa.6
CTIVP, computed tomography intravenous pyelography; CTKUB, computed tomography of the kidneys, ureters and bladder; urine MCS, urine microscopy/culture/sensitivities; USKUB, ultrasound of the kidneys, ureter and bladder; UTI, urinary tract infection.
Risk factors for rUTIs (Figure 1) are similar to risk factors for acute UTI. rUTIs are often caused by various factors such as the impaired host immunity or presence of structural or functional abnormalities of the urinary tract.6,9–11 Without due attention and mitigation, patients with risk factors are more likely to develop rUTIs.
Diagnostic evaluation
We present a summary for current diagnostic and management considerations for rUTIs (Figure 1).
Aim
A narrative review guided by expert opinion was conducted to provide a targeted overview of contemporary management strategies for recurrent urinary tract infections.
Results and Discussion
Non-surgical treatment
Behavioural modifications
Most guidelines encourage increasing fluid intake to greater than 1.5 L/day to reduce the number of UTIs and subsequent antibiotic treatment required. This is thought to be mediated by diluting bacterial concentration in the bladder.6,17 Other behaviours to reduce risk factors for rUTIs include wiping from front to back after defecation and post-coital urination, which are thought to help by reducing female periurethral colonisation.2,3,17 These behavioural modifications, although not strongly supported by evidence, have a limited adverse effect profile, and can offer benefits to some patients. With this in mind, behavioural modifications can be included in the counselling of patients regarding rUTIs, but they do not need to be central in the discussion. Patients should be reassured that their hygiene practices do not place them at fault when experiencing rUTIs.17
Non-antibiotic treatments
Second-line treatment for rUTIs include non-antimicrobial prophylaxis (Table 2); these can be used according to the suggested treatment guideline (Figure 2).
| Table 2. Summary of non-antibiotic treatments for recurrent urinary tract infections (rUTIs) |
| Intervention |
Mechanism of action |
Side effects |
Limitations |
Summary |
Level of evidence6 |
Action based on summary of international guidelines17 |
| Non-antibioitic (oral and vaginal) therapies |
Vaginal topical oestrogen replacement
|
Reduced vaginal flora colonisation by depleting glycogen18 |
Vaginal bleeding, vaginal discharge, vagina irritation18 |
- Small studies reporting heterogenous treatment schedules18
|
- Found to reduce rUTI in postmenopausal patients18
- Common misconception regarding cardiovascular and cancer risk associated with long-term oestrogen; however, it is supported for use in postmenopausal patients 17
|
1b |
Recommended |
Methenamine hippurate
|
Hydrolysed in the bladder to form formaldehyde for bactericidal effects1,6,19 |
Non-specific19 |
- Not effective in patients who have renal tract abnormalities or neuropathic bladder19
|
- Effective in preventing UTI when used as prophylaxis,6 although long-term benefit is limited with the same rate of recurrence after 1 year9,19
- Found to be non-inferior in prevention of UTI compared to daily continuous low-dose prophylaxis20
- Unlike prolonged antibiotics, patients are unlikely to develop resistance over time19,20
|
1b |
Recommended |
| Cranberry |
Contain proanthocyanins that stop bacterial adherence to bladder mucosa2,4,5,21 |
Non-specific gastrointestinal symptoms21 |
- No standardised dosage or duration of treatment reported in literature21
- Evidence comparing the efficacy between different forms of cranberry (eg juice, supplementation) is limited21
|
- Efficacious in women and children only4,21
|
1a |
Consider |
| D-mannose |
Prevention of bacterial adherence to the bladder mucosa4,22 |
Non-specific gastrointestinal symptoms and vaginal irritation22 |
- No standardised dosage or duration of treatment in literature22
- Lack of high-quality randomised control trials
|
- There is contradictory evidence regarding the role of D-mannose in the treatment of rUTI22
|
2 |
Consider |
Probiotics
(Most commonly, Lactobacillus) |
Converts glucose to lactic acid to acidify urine for a more hostile environment for uropathogens4,5,23 |
Non-specific23 |
- Possible benefits reported in very small studies with poor methodological reporting and heterogeneity in the type of probiotic used6,23
|
- No significant benefits supporting use of probiotics compared with placebo or no treatment23
|
1b |
Not supported |
| Intravesical and novel therapies |
Intravesical glycosaminoglycan therapy (eg hyaluronic acid and chondroitin sulfate)
|
Replenishes pre-existing non-stick coating on bladder mucosa3,24 |
Mild bladder pain, risk of UTI given catheterisation required |
- Two randomised control trials have shown promising results, with decrease in UTI recurrence, greater time between UTI recurrence, improvement in quality of life24,25
|
- More research required before recommendation for intravesical glycosaminoglycan therapy can be made6
|
2 |
Consider |
Immunoactive agents and vaccines
|
Contains bacterial extracts to stimulate excretion of cytokines from urothelium, and have different routes of administration: oral (OM-89, MV140), vaginal pessary, (MV140), injection (StroVac)4,6
|
Dependent on method of administration |
- Restrictions on availability in Australia and requires urologist to coordinate access
- Low number of studies within each mode of administration6
- Development of direct vaccine targets to bacterial antigens are currently in testing phase4
|
- Vaccine prophylaxis has been found to be more effective compared to placebo, although there are only a low number of studies within each mode of administration6
|
1a |
Recommended |
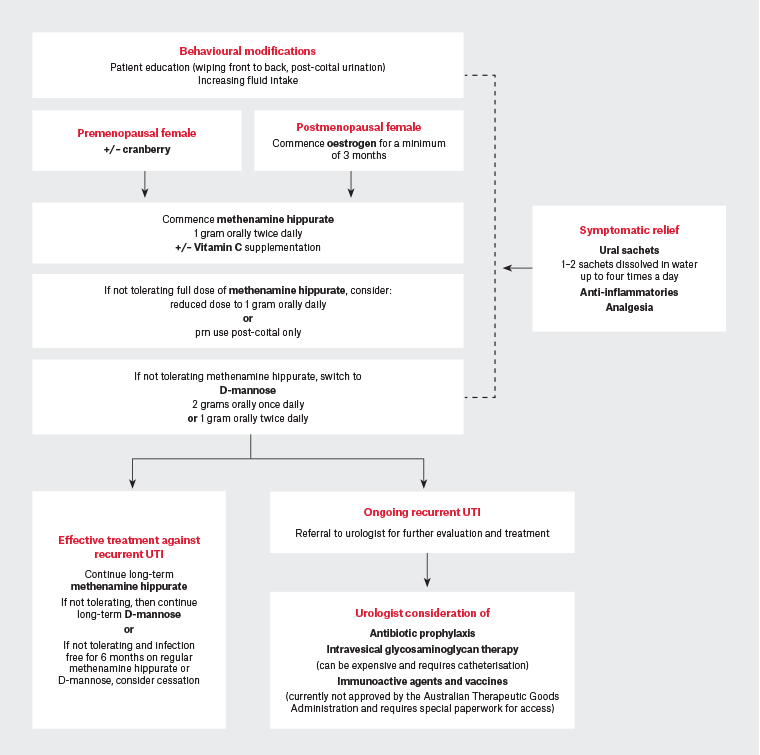
Figure 2. Suggested management algorithm for recurrent urinary tracts (rUTIs). Click here to enlarge
Antibiotic treatments
Active UTIs should be treated empirically, with rationalisation to more directed antibiotic treatment once urine culture results become available.9 Prevention of future episodes with antibiotic prophylaxis (Table 3) is supported by all guidelines and needs to be offered to those patients suffering from rUTIs.17 If antibiotic prophylaxis is being considered, prompt urology referral is required to consider other non-antibiotic measures. Prophylactic antibiotics can be associated with more side effects (eg nausea, diarrhoea, vaginal candidiasis) compared to non-antibiotic measures.20 Routine post-treatment urine MCS in asymptomatic patients is not required.6
| Table 3. Treatment regimen for antibiotic prophylaxis28 |
| Treatment regimen |
Duration |
Antibiotics used |
Dosing regimen |
| Post-coital prophylaxis |
|
- Trimethoprim 150 mg orally
- Cephalexin 250 mg orally
- Nitrofurantoin 50 mg orally
|
- Single dose of antibiotic within 2 h post intercourse
- Patients should swap agents every 6 weeks
- If patients remain infection free for 6 months with post-coital prophylaxis treatment, antibiotic prophylaxis can cease with a trial of post-coital high-dose cranberry
|
| Patient-initiated treatment |
- Standard short course of therapeutic antibiotics
|
- Trimethoprim 300 mg orally daily for 3 days
- Cephalexin 500 mg orally 12 h for 5 days
- Nitrofurantoin 100 mg orally 6-hourly for 5 daysA
|
- Patient should provide urine sample for MCS prior to commencement of antibiotics (to monitor for resistance to prescribed antibiotics; not to confirm UTI)
- Antibiotics should be rationalised once culture results are available (note: commonly used antibiotics will not treat Enterococcus UTI, and amoxicillin is required to treat)
|
| Continuous low-dose prophylaxis |
- 6 months with review at 3 months
- Potentially life long if relapse after cessation
|
- Trimethoprim 150 mg orally nightly
- Cephalexin 250 mg orally nightly
- Nitrofurantoin 50 mg orally nightlyA
|
- Choice of antibiotic depends on previous urine cultures, local antibiogram and patient tolerability
|
AMonitoring must be undertaken for long-term nitrofurantoin treatment due to risk of rare adverse effects including pulmonary toxicity, hepatotoxicity and peripheral neuropathy.
MCS, urine microscopy/culture/sensitivities; UTI, urinary tract infection. |
Antibiotics in pregnancy
Recurrent urinary tract infection in pregnancy is defined as having two or more UTIs diagnosed during pregnancy, and occurs in 4–5% of pregnancies.7 Asymptomatic bacteria and UTI in pregnancy should be treated and referred to a urologist due to their association with adverse pregnancy outcomes for both mother and child.26 Pregnant patients are more likely to develop pyelonephritis from asymptomatic bacteriuria or UTI. Asymptomatic bacteriuria and UTI in pregnancy is also associated with preterm delivery and low birth rate.7,27 Patients should have their acute episode of UTI treated with appropriate antibiotics, and might require post-coital or continuous prophylaxis for the remainder of their pregnancy, with antimicrobial choice dependent on what is safest during pregnancy.7
Post-coital prophylaxis
For those with rUTIs clearly associated with sexual activity, a single dose of antibiotic post-intercourse has been found to be just as effective as low-dose, long-term prophylaxis (Table 3).2,17,28
Self-directed prophylaxis
Self-directed prophylaxis is appropriate for patients who are able to initiate testing and treatment when the first symptoms of a UTI present. These patients should be encouraged to provide a urine sample before commencing treatment.1 Self-directed prophylaxis is as efficacious as continuous low-dose prophylaxis and has fewer anti-microbial gastrointestinal-related side effects (Table 3).9
Continuous low-dose prophylaxis
If patients fail self-directed treatment, low-dose, continuous antibiotic treatment might be required.2,28 An initial course of six months with three-monthly review for efficacy and side effect profile is appropriate (Table 3).3 For some patients, treatment can be lifelong, as cessation of continuous antibiotic prophylaxis can lead to rUTI relapse. Antibiotic selection should be based on urine cultures and the local antibiogram,2 with a lower dose used compared to that used in the treatment of acute cystitis.9 Patient tolerability of antibiotics might also have an impact on the choice of antibiotics selected, and patients need to be monitored for potential adverse reactions to long-term antibiotic use.9,29 Only a low number of patients require this type of regimen, and it is best for treatment to be specialist-directed in these cases.
Surgical interventions
It is rare to find a reversible surgical cause in patients with rUTIs, with the exception of pelvic organ prolapse or urinary tract obstruction. Patients with infection refractory to simple measures, as outlined above, should be referred for urologist review, especially in the event of any red flags such as a history of urinary tract surgery including incontinence procedures with mesh, abnormal ultrasound findings or underlying neurology. Surgical interventions aim to remove the underlying infective aetiology.30
Sources of infection can include foreign bodies such as stents, erosive mesh/sutures or renal/bladder calculi, which can all act as nidi for persistence. Most foreign bodies can be removed endoscopically. Urinary fistulae can also be a source of reinfection and can be managed with minimally invasive or open surgery.30 Malignancy can be treated endoscopically with transurethral resection of a bladder tumour. In patients with urinary stasis secondary to bladder outlet obstruction, the cause needs to be accurately identified and treated.9,30
If pelvic organ prolapse is the cause of bladder outlet obstruction, surgical treatment might be indicated and a referral to a practitioner who practises in prolapse management, such as a urologist or gynaecologist, is recommended. In males, surgery for benign prostatic enlargement or high bladder neck, which can often affect emptying of the bladder leading to infection, is offered following failure of medical therapy.2,9,30 Dilation of strictures or urethroplasty can be considered if urethral stricture disease is suspected to have caused urinary stasis.30 Urinary stasis due to urinary diverticulum might require diverticulectomy. Neuropathic bladder needs to be addressed appropriately to minimise rUTIs and preserve upper tract function.30
Conclusion
rUTIs can be effectively managed in the general practice setting. Early effective management is hoped to reduce side effects, antimicrobial resistance and, most importantly, long-term suffering for these patients. However, careful history taking, examination and investigation are essential to rule out reversible causes before committing to long-term therapies.
All males with rUTIs should be referred for specialist opinion. Females with uncomplicated rUTIs and without clinical red flags can have their treatment tailored to replace oestrogen in postmenopausal females or trial an oral non-antibiotic agent, both of which are highly effective and low-risk strategies. Referral to urologist can be made for any additional doctor or patient concerns, although a trial of these treatments can improve or even completely resolve patient symptoms while they await specialist review.
Although antibiotic prophylaxis can control rUTIs, proper patient education about preventive measures such as behavioural modifications are necessary to reduce rUTIs, along with simple non-antibiotic measures.
Urology review can be sought for consideration of long-term antibiotic prophylaxis strategies, intravesical therapies and novel immunoactive agents such as vaccines.
Key points
- Patient education, preventive measures and behavioural modifications can reduce rUTIs.
- Identified risk factors for rUTIs should be addressed.
- Referral to a urologist should be made for an assessment if there are concerns about underlying conditions leading to infection or ‘red flags’ in females and for all males.
- General practitioners should be supported in commencing treatments for rUTIs while a patient is awaiting urological review.
- There is a role of shared care for patients who require escalation of rUTI treatment to long-term prophylactic antibiotics, intravesical therapies and novel immunoactive agents.