Case
A man aged in his late 70s presented with an 11-year history of a pigmented lesion on his left cheek, which he reported has recently changed in size and colour. There is scarring and hypopigmentation in the area secondary to previous excision of a basal cell carcinoma (BCC) and use of imiquimod for solar keratosis many years earlier.
The patient is of Fitzpatrick skin type 2 with a lifelong history of recreational sun exposure, multiple facial and trunk BCCs and no history of melanoma. Medical history includes type 2 diabetes, benign prostate hyperplasia, polymyalgia rheumatica, hypertension, peripheral vascular disease and macular degeneration; the patient is an ex-heavy smoker and lives alone.
Question 1
On examination there is an asymmetrical, variably pigmented patch with background actinic damage on the left cheek measuring 50 mm×45 mm (Figure 1). Dermoscopy reveals a non-uniform lesion coloured brown, grey, pink and white, with an irregular structure, annular granular pattern, rhomboid structures, grey dots/concentric circles and structureless areas (Figure 2).
What is your favoured diagnosis? What differentials might be considered?
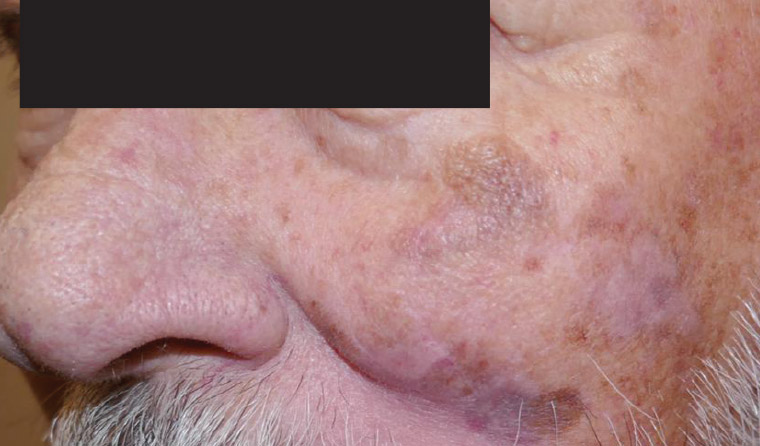
Figure 1. Pigmented facial lesion.
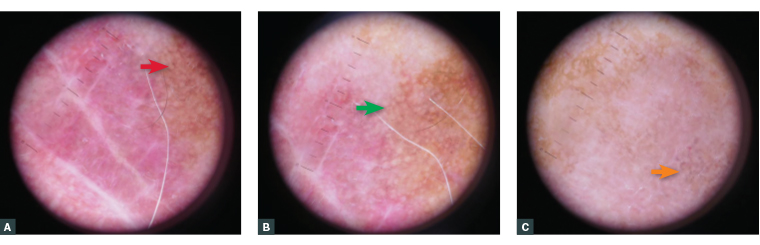
Figure 2. Dermoscopic features of the lesion. (A) Rhomboid structures (red arrow). (B) Structureless areas (green arrow). (C) Grey dots/grey concentric circles (orange arrow).
Question 2
What would the initial management involve?
Answer 1
The most likely diagnosis is lentigo maligna (LM), an in situ melanoma confined to the epidermis, or lentigo maligna melanoma (LMM), where it has become invasive.
Typical features of differential diagnoses are provided in Table 1.
| Table 1. Typical features of differential diagnoses |
| Condition |
Macroscopic features |
Dermoscopic features |
| Lentigo maligna/lentigo maligna melanoma |
- Often >6 mm, irregular shape, variable pigmentation, tan, light and dark brown, pink, red and white colours, with a smooth surface
|
- Asymmetrical pigmented follicular openings, rhomboid structures, grey pseudonetwork, annular granular pattern, grey dots and globules4
|
| Pigmented intraepidermal carcinoma/Bowen’s disease |
- Typically irregular scaly macular to raised lesions
- Might be several centimetres in diameter, orange–red or brown in colour, with areas of pigmentation and smooth, hyperkeratotic or ulcerated
|
- Dots, structureless areas, dots in linear arrangement, coiled vessels4
|
| Pigmented actinic keratosis |
- Flat or thickened papule or plaque
- Pigmented or white, yellow or red
- Scaly, warty or horny surface
|
- Hyperpigmented follicular openings, brown structureless areas, annular–granular structures
- Occasionally angulated superficial brown lines
- Perifollicular inner grey halo, grey rhomboidal structures4
|
| Solar lentigo |
- Flat macule or patch, multiple colours, including yellow, light or dark brown, regular or irregular border, dry surface, well circumscribed, moth-eaten outline
|
- Faint pigment network, fingerprint structures, uniform pigmentation, elongated rete ridges4
|
| Lentiginous nevus |
- Irregular borders, multiple colours, including pink, red, tan, brown and black, and a diameter usually >5 mm
|
- Reticular, globular, asymmetrical pigmentation, regression structures, irregular vascular pattern, blue-grey areas4
|
| Melasma |
- Bilateral, blotchy, brownish facial pigmentation, light-to-dark brown macules or patches with irregular borders
|
- Light to dark brown reticular network, brown dots, granules and globules, arcuate and annular structures, sparing of perifollicular region and openings of sweat glands, arcuate and annular pattern4
|
| Seborrhoeic keratosis |
- Well demarcated, flat or raised, waxy papules and patches, central verrucous changes
- Skin coloured, yellow, grey, light brown, dark brown, black or mixed colours
|
- Comedo-like openings, milia-like cysts, hairpin vessels, fingerprinting, moth eaten and sharply demarcated, network-like structures, pseudonetwork4
|
Pigmented basal cell carcinoma
|
- Areas of pigmentation, nodular or plaque, pearly appearance, telangiectasia, central ulceration
|
- Leaf-like structures, blue-grey ovoid nests, spoke-wheel areas, blue-grey globules, arborising telangiectasia and ulceration4
|
| For those interested in a more comprehensive comparison, see Tiodorovic-Zivkovic et al.5 |
Answer 2
Clinical examination for lymphadenopathy and hepatosplenomegaly is crucial. LM can harbour significant subclinical extension and occult dermal invasion.
Recommended initial management for suspected melanoma is excisional biopsy with 2-mm margins.1 Due to the size and location of this lesion, a central incisional biopsy of the lesion was performed (see below), alternatives being multiple punch/shave scouting biopsies (any partial biopsy carries risk of false negatives).1,2
Determination of lesion extent is crucial to management planning. Techniques, including dermoscopy, Woods light examination and mapping biopsies, can aid lesion delineation, and were subsequently performed (see below). Confocal microscopy might be used for this purpose but has limited availability.3
Case continued
An incisional biopsy, 18 mm×10 mm×8 mm, showed Clark Level 1 (in situ melanoma) of the LM type. Melanin incontinence was present, with no evidence of adnexal extension or dermal invasion.
To determine lesion extent, four 2-mm diameter circumferential mapping punch biopsies were taken 10 mm from the lesion. The left inferior specimen showed LM. The other biopsies showed actinic damage and basal layer hyperpigmentation associated with a focally lentiginous pattern.
Question 3
What are the management options for this patient?
Answer 3
The clinical situation is poorly defined LM on the left cheek, where invasive disease has not been excluded, in an elderly patient with multiple medical comorbidities. First-line treatment is excision with 5- to 10-mm margins, although lesions larger than 10 mm or on the head and neck might require a wider margin.3,6 Recurrence rates for excised LM are approximately 6% at five years, but the risk of recurrence increases for lesions with a large diameter and/or those that are poorly defined.7
Excision options for this patient are as follows:
- excision with a repair that enables re-excision in the event of histological margin involvement3
- excision with margin control (eg Mohs surgery or staged excision with delayed definitive repair).4,7
If surgery is refused or contraindicated, options include:
- radiation treatment8,9
- imiquimod10,11
- careful observation (only in selected cases with very elderly patients, or those with significant comorbidities and after review at multidisciplinary clinic with informed consent).12
Alternative treatment options (Table 2) might be discussed.
| Table 2. Non-surgical treatment options |
| Treatment modality |
Recurrence rate |
Advantages |
Disadvantages |
| Radiation therapy |
- Mean recurrence estimate of 11.5%13 or recurrence estimate of 5% in 3 years with electron-specific treatment8
|
- Titration to adequate depth13
- Treat undetected invasion13
- Nil general anaesthetic or surgery
|
- Multiple treatments
- Short-term side effects:
- Pain
- Fatigue
- Erythema
- Desquamation
- Mucosal irritation (not this particular patient)
- Long-term side effects:
- Radiation-induced optic neuropathy (not this particular patient)
- Alopecia
- Telangiectasia
- Hyperpigmentation
- Secondary malignancy
|
| Imiquimod |
- Mean recurrence estimate ranges between 0% and 40%14,15
|
- Non-invasive
- Patient-controlled home treatment
|
- Lack of long-term outcome data16
- Might not treat adnexal extension or occult invasion
|
Case continued
The patient refused surgery.
Question 4
What would be your response in this situation?
Answer 4
When a patient declines optimal treatment, their reasons must be thoroughly and sensitively examined. Potential barriers include procedural fear, previous poor surgical experience, concerns about scarring, concerns regarding general anaesthesia and cost. Factors to consider include allowing time in the consultation for all questions to be addressed, a support person to be present and organising patient follow-up. Comprehensive documentation of the discussion and patient consent are crucial.17
Case continued
The patient was reviewed at a multidisciplinary clinic (plastic surgery, radiation oncology and dermatology) where excision was strongly recommended. Despite counselling, the patient declined, citing his age, frailty, comorbidities, lesion location, unwillingness to endure a substantial procedure and firm personal beliefs about illness and medicine. He was advised that surgical treatment had the highest cure rate and that the lesion could progress to invasive malignancy and metastatic disease. He understood these risks, and a support person attended appointments with him.
The patient opted to trial imiquimod treatment encompassing 10 mm outside the lesion margins. An adequate inflammatory response was achieved for 12 weeks. Current guidelines suggest daily use for five days a week for 12 weeks.11 Unfortunately, there was a poor clinical response, with dermoscopically evident extensive residual disease at review four weeks after treatment completion.
A six-week course of radiation treatment was prescribed. The patient experienced short-term mild erythema, dry desquamation and mild irritation to the treatment field managed with emollients. Mild hyperpigmentation was the only late toxicity noted.
There was complete clinical and dermoscopic clearance 12 weeks after treatment, and the patient remains free of any sign of local or systemic recurrence at the three-year follow-up (Figure 3).
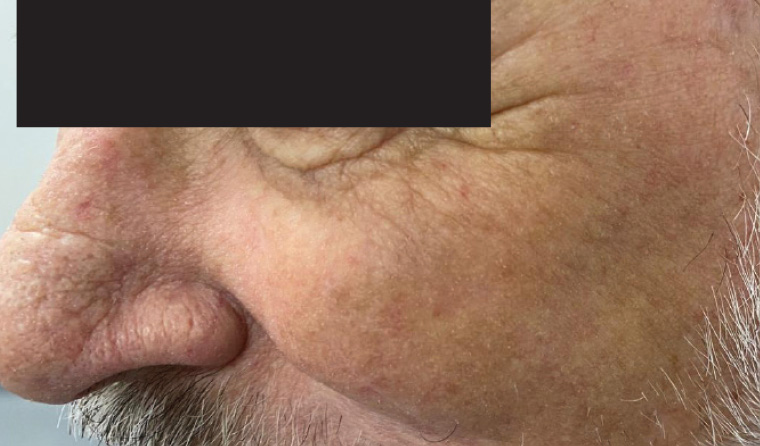
Figure 3. Clinical and dermoscopic clearance at 12 weeks’ post treatment.
Key points
- Large-diameter LM on sites of functional and cosmetic importance presents a difficult management issue.
- Surgical excision remains first-line treatment. When this is not possible due to lesion extent, comorbidities or patient preference, the patient should be counselled as to the available options and their relative risks and benefits.
- Radiation therapy, imiquimod or careful observation in selected patients are alternatives if surgery is not possible or declined.
- Strategies to support patients’ autonomous and informed decision-making process include adequate consultation time, the facilitation of a support person and regular follow-up sessions.