Background
The widespread use of cross-sectional imaging has led to the increased detection of urological incidentalomas. Incidental renal and adrenal masses are the most commonly detected urological incidentalomas and are often encountered by general practitioners.
Objective
This review aims to provide an evidence-based approach to managing renal and adrenal masses.
Discussion
Renal lesions occur in 14% of computed tomography (CT) scans. Differentials include cysts (benign or malignant), angiomyolipomas, oncocytomas and renal cell carcinomas (RCCs). The Bosniak classification should be used for cystic renal lesions. Active treatment should be considered for RCCs that are >4 cm, symptomatic or rapidly growing. Patients with adrenal lesions should undergo functional work-up. If clinically concerned, screening tests include 1 mg overnight dexamethasone suppression test and plasma or urinary metanephrines. In the presence of hypertension or hypokalaemia, screening for hyperaldosteronism with the plasma aldosterone-to-plasma renin ratio should be considered. Benign adrenal adenomas on CT are <4 cm, homogenous and hypodense (Hounsfield unit <10).
Widespread use of cross-sectional imaging has resulted in increased detection of urological incidentaloma. Renal and adrenal masses are the most common, respectively accounting for 14% and 5% of incidentalomas in patients undergoing computerised tomography (CT).1,2 Incidentalomas pose a diagnostic challenge, balancing between over-investigation and misdiagnosis.
Aim
This clinical review aims to provide an approach for the management of renal and adrenal incidentalomas.
Approach to renal incidentaloma
Renal cyst
The incidence of renal cystic disease increases with age, with most cysts being benign.3 The Bosniak classification is used to characterise renal cysts, predict malignancy risk and guide treatment (Table 1).4–7 Contrast-enhanced CT (CECT) is helpful when uncertainty exists regarding the classification of a non-contrast CT. Bosniak I (simple) and Bosniak II cysts are benign and do not require further follow-up.7 Bosniak IIF cysts harbour a 5–10% risk of malignancy, and 12% of them progress to Bosniak III/IV cysts during follow-up.7,8 Patients with Bosniak IIF cysts should undergo active surveillance (AS) with six-monthly scans initially, extending up to 12-monthly for a total of five years. The modality of scans is dependent on tumour location, body habitus, radiation exposure and renal function. Patients with stable Bosniak IIF cysts can undergo surveillance by general practitioners (GPs). Triggers for re-referral to a urologist are increase in size, symptoms or concerns of metastatic disease. Bosniak III and IV cysts harbour a higher risk of malignancy (50% and 90–100%, respectively) and should be referred to a urologist with a category 2 classification (seen within 90 days) if the lesion is <4cm. A category 1 (seen within 30 days) referral should be made if the lesion is >4 cm or if there are concerns of metastatic disease. Although AS of Bosniak III might be an option, the decision should be made by a urologist in association with a multidisciplinary team.
| Table 1. Simplified summary of Bosniak classification, version 20194–6,8 |
| |
Class |
Estimated risk of malignancy (%) |
Radiological features |
Recommendations |
| Simple |
I |
0 |
Thin, smooth wall (≤2 mm), might enhance, no septa, no calcification, no solid component |
No follow-up |
| Complex |
II |
0 |
Thin (≤2 mm) and few smooth septa (1–3) ± calcification
or
Hyperattenuating (HU>20), non-enhancing, small (≤3 cm) and homogeneous |
No follow-up |
| IIF |
5–10 |
Many (≥4), thin (≤2 mm) septa that must enhance
or
Minimally thickened (3 mm) septa that must enhance
or
Cystic masses at MRI that are heterogeneously hyperintense on fat-saturated unenhanced T1-weighted imaging that do not meet criteria for Bosniak III or Bosniak IV |
Surveillance (US or CT, initially 6-monthly, extending up to 12-monthly for a total of 5 years)
Referral to urologist if symptomatic, unclear diagnosis, or concerning of metastasis |
| III |
50 |
≥1 thick (≥4 mm) or irregular (≤3 mm focal or diffuse protrusion with obtuse margin with wall or septa) enhancing walls or septa without nodular enhancement |
Referral to urologist for consideration of surgery or active surveillance in selected casesA |
| IV |
90–100% |
≥1 enhancing nodule (focal enhancing convex protrusion of any size that has acute margins with the wall or septa or a focal enhancing convex protrusion ≥4 mm) |
Referral to urologist for consideration of surgery |
Radiological features are based on contrast-enhanced CT unless specified otherwise.
AThe decision for active surveillance should be made by a urologist and associated multidisciplinary team.
CT, computed tomography; HU, Hounsfield unit; US, ultrasound. |
Angiomyolipomas
Angiomyolipomas (AML) are benign mesenchymal neoplasms.9 Oestrogen contributes to their development; therefore, their predilection is towards females. AMLs are often asymptomatic but could present with pain or haemorrhagic complications. Risk factors for haemorrhage include tumour size, vascularity and periods of elevated oestrogen (pregnancy or exogenous hormone). Asymptomatic AML <2 cm might not require follow-up due to the low risk of haemorrhage (Figure 1). AML between 2 and 4 cm can be considered for AS. Currently, there is no consensus on the ideal AS protocol. A common approach involves ultrasound every 6–12 months for up to two years and to consider discharge if stable.10 AS over a two-year duration appears to provide the same benefit as follow-up over five years.10 Frequency and duration of AS should be tailored to patients’ comorbidities, age and risk of bleeding. Patients with a stable AML can undergo surveillance by GPs, and a non-urgent urology referral can be made to clarify follow-up. Treatment should be considered if patients experience pain, recurrent haemorrhage or are at risk of haemorrhage (AML >4 cm or progressive in size or the patient is planning for pregnancy).6,11 Treatment options include selective arterial embolisation (SAE) or nephron-sparing surgery (NSS), such as partial nephrectomy. SAE is less invasive but associated with higher re-treatment rates compared to surgery (31% vs 0.85%).11 AML in a woman of childbearing age or ≥4 cm can be referred to a urologist as category 2.
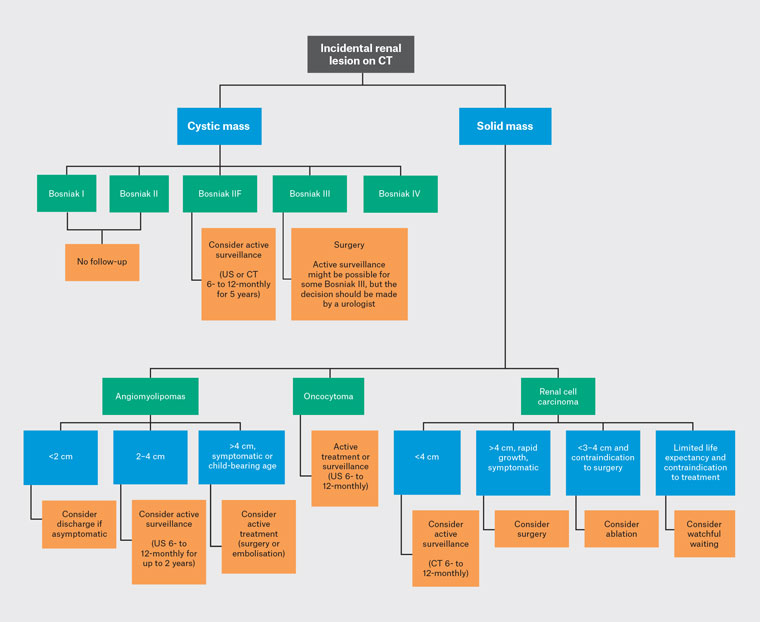
Figure 1. Simplified diagnostic algorithm for incidental renal lesion on CT.
CT, computed tomography; US, ultrasound.
Oncocytoma
Oncocytomas are benign and present in the sixth to seventh decade of life.12 Oncocytomas are radiologically challenging to differentiate from renal cell carcinomas (RCCs) as they present as solid masses within the renal cortex. CT and magnetic resonance imaging (MRI) have limited utility for diagnosis, and histopathological confirmation is preferred. Any solid renal mass should be referred to a urologist as category 2 if <4 cm or category 1 if >4 cm. Treatment options include surgery or AS in selected biopsy-proven oncocytoma because of the risk of harbouring chromophobe RCC.6,13 AS consists of 6- to 12-monthly scans with either CT or ultrasound.14 A clear AS plan should be obtained from a urologist as oncocytomas have a median growth of
1.5 mm/year.
Renal cell carcinoma
The incidence of RCC has been increasing. Risk factors include tobacco use, obesity and family history of RCC.15,16 Most RCCs are asymptomatic and incidental; the triad of flank pain, macroscopic haematuria and palpable abdominal mass is now rare due to earlier detection.17,18 Referral to a urologist is warranted: as category 2 if <4 cm or as category 1 if >4 cm or there are concerns of metastatic disease. The decision for renal biopsy should be made by a urologist and is reserved for patients planned for ablative therapy or if diagnostic uncertainty remains. Renal biopsies are avoided for a cystic mass due to lower diagnostic yield.6 Treatment options for localised RCC include AS, surgery, ablation or watchful waiting (WW). AS is reserved for patients with good life expectancy and renal mass <4 cm, as risk of metastasis is only 1.8% at this size.19 CT is preferred and performed initially six-monthly extending to 12-monthly if stable. Surgery is performed minimally invasively (laparoscopic or robotic) and NSS (partial nephrectomy) whenever possible. Ablation is typically reserved for smaller lesions <3–4 cm. Ablation could be considered based on patient preference or in patients with good life expectancy but not suitable for surgery. Ablation has low complication rates, but data is lacking on long-term oncological outcomes compared to surgery.20 WW is reserved for comorbid patients with limited life expectancy where the risks of active treatment outweigh the benefits. Patients are followed up symptomatically and follow-up imaging might not be required.
Approach to incidental adrenal incidentaloma
Adrenal incidentaloma can be categorised as benign nonfunctional, benign hyperfunctional or malignant (Figure 2). The initial diagnostic consideration is whether the adrenal lesion is unilateral or bilateral. Bilateral adrenal lesions account for 10% of cases and can be associated with benign adenomas, infections (eg fungal or tuberculosis), congenital adrenal hyperplasia, lymphoma or metastatic disease.21 Some primary malignancies (eg of lung, colorectal, breast, pancreas or renal origin) might metastasise to either unilateral or bilateral adrenal glands. However, the presence of non-adrenal primary malignancies does not rule out benign adrenal adenomas.
The second consideration is whether the lesion is functional. Benign nonfunctional adenomas account for 80% of adrenal incidentalomas. Functional adrenal lesions are mostly benign and are made up of 5% phaeochromocytoma (PCC), 5% cortisol secreting and 1% aldosterone secreting.22 The remaining consist of primary malignancy and adrenal metastasis.
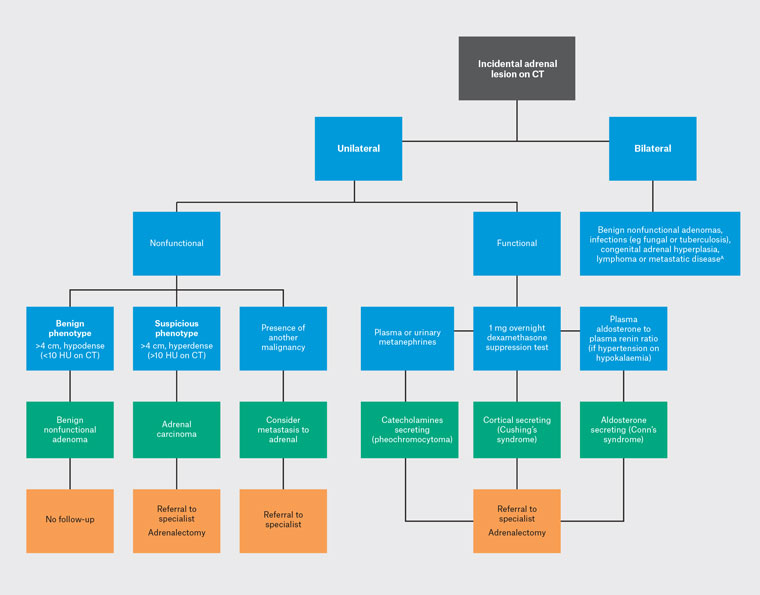
Figure 2. Simplified diagnostic algorithm for incidental adrenal lesions on CT.
AMetastatic disease can be either unilateral or bilateral.
CT, computed tomography; HU, Hounsfield unit; US, ultrasound.
PCCs secrete adrenaline and noradrenaline. Patients with PCCs can present with palpitation, headaches, tremors, sweating, weight loss and hypertension. This could be screened with plasma or urinary metanephrines.23 Cortisol-secreting adrenal mass results in Cushing’s syndrome, characterised by moon face, buffalo hump, central obesity, hypertension and diabetes. Screening can be conducted with a 1 mg overnight dexamethasone suppression test and confirmed with an 8 mg dexamethasone suppression test or midnight salivary cortisol.23 An aldosterone-secreting adrenal mass will manifest as Conn’s syndrome, which is often asymptomatic but can present with refractory hypertension or hypokalaemia. This could be screened with plasma aldosterone to plasma renin ratio and confirmed with 24-hour urinary aldosterone.22,23 In the primary care setting, all patients with adrenal incidentaloma should undergo clinical assessment for signs and symptoms of hormone excess. If hyperfunctioning adrenal lesion is suspected, GPs can screen with the 1 mg overnight dexamethasone suppression test and plasma or urinary metanephrines. Screening with the plasma aldosterone-to-plasma renin ratio should be reserved for patients with hypertension or unexplained hypokalaemia. Confirmatory tests should be deferred to a specialist (eg 8 mg dexamethasone suppression test or midnight salivary cortisol). All functional adrenal incidentalomas should be referred for specialist review and classified as category 1.24 Medical management is performed by an endocrinologist, and adrenalectomy is typically performed by an endocrine or urology surgeon.
Finally, benign adrenal masses exhibit three radiological features: they are <4 cm in size, homogeneous and hypodense (<10 Hounsfield unit [HU]). Risk of adrenal malignancy increases with size: the risk is 5% for masses <4 cm, 10% for those 4–6 cm and 25% for those >6 cm.23 Benign adrenal adenomas contain intracytoplasmic fat and appear hypodense on non-contrast CT.25 If non-contrast CT is consistent with a benign adrenal adenoma, no further imaging is required. However, up to 30% of adenomas are lipid poor and might not appear hypodense. In these situations, a CECT with adrenal protocol might be helpful. This involves a delayed-phase CECT to determine the rate of contrast washout.26 Both benign adenomas and carcinomas enhance rapidly with contrast, but benign adenomas typically exhibit a faster contrast washout compared to carcinomas. MRI is comparable to non-contrast CT for identifying intracytoplasmic fat; however, it is comparable to washout CT scans.22 Specialist referral is recommended if the diagnosis remains unclear or if there are concerns of adrenal malignancy. Benign adrenal adenomas do not require follow-up or specialist referrals if <4 cm.
Conclusion
If a renal incidentaloma is found on an ultrasound or non-contrast CT and the diagnosis is unclear, consider a CECT. Bosniak I and II renal cysts do not require further imaging. Non-contrast CT might be sufficient to exclude a malignant adrenal lesion. CECT with adrenal protocol can be helpful if the diagnosis is unclear.
Key points
- Renal cysts should be characterised using the Bosniak classification.
- Bosniak I (simple) and Bosniak II cysts do not require further work-up or treatment.
- Solid renal masses should be referred for urological opinion; urgency will depend on size and symptoms.
- Adrenal incidentalomas should undergo functional assessment, which includes clinical evaluation, and, if necessary, endocrinological screening tests.
- If non-contrast CT of adrenal incidentaloma have benign features (ie <4 cm, homogenous, and hypodense [HU<10]), no further imaging or follow-up is required.