Comments on ‘Melanoma imaging and diagnosis: What does the future hold?’
In Australia, the growing application of artificial intelligence (AI) in skin cancer diagnosis is a significant advancement, as early detection is critical to improving survival rates. This article ‘Melanoma imaging and diagnosis: What does the future hold?’ by von Schuckmann et al investigates current advances in imaging and diagnostic approaches, with a special emphasis on the function of AI in tracking changes in moles, particularly atypical nevus, which are known to have high risk for skin cancer.1 The use of digital examination techniques demonstrates the potential to reduce needless biopsies for patients with high nevus counts, hence enhancing patient care.
One obvious flaw in the current application of AI in skin cancer detection is the variation in algorithm accuracy between skin kinds and backgrounds. Much of the research relies heavily on population similarities, which might render them inapplicable to a larger group.2,3 Furthermore, despite AI’s tremendous potential for detecting malignant alterations, dependence on the technology might diminish clinician contact with patients and self-examination studies. Furthermore, questions about data privacy and security of medical data handled by AI systems have received little attention in academic literature.
Despite the potential benefits of AI technology, there are currently no formal standards for integrating it into existing healthcare practices. However, there has been little discussion on the necessity for standardised training for healthcare professionals to efficiently use different AI technologies. Furthermore, the potential use of telemedicine mixed with AI for remote monitoring of high-risk patients has not received significant attention, particularly in rural Australia, where access to a dermatologist might be limited.
Looking ahead, research should focus on building more comprehensive datasets that cover a wider range of skin types and environmental factors. Collaboration between technology developers and dermatologists could produce AI systems tailored to different populations.
Furthermore, the creation of protocols for hybrid models that combine AI technology with physician assessment could increase diagnostic accuracy while retaining the essential human element of patient care. Long-term studies tracking outcomes for patients monitored with AI tools could also provide valuable insights into patient efficacy and satisfaction.
This strategy is unique in that it combines AI with whole-body imaging techniques, rather than just targeted tumour surveillance. This comprehensive approach could considerably enhance melanoma management, allowing physicians to more correctly identify tumour alterations while minimising the number of unnecessary surgical procedures. The ability of AI-powered technologies to give real-time analysis and notify physicians to concerning changes in a patient’s skin condition marks a fundamental shift in how melanoma is diagnosed and managed, emphasising the importance of technology in healthcare.
Author
Amnuay Kleebayoon PhD, Private Academic Consultant, Samraong, Cambodia
Viroj Wiwanitkit MD, Saveetha Medical College and Hospital, Saveetha Institute of Medical and Technical Sciences, Saveetha University, Tamil Nadu, India
Competing interests: None.
References
- von Schuckmann L, Banney L, Soyer HP. Melanoma imaging and diagnosis: What does the future hold? Aust J Gen Pract 2024;53(9):633–34. doi: 10.31128/AJGP-02-24-7156.
- Yuan L, Jin K, Shao A, et al. Analysis of international publication trends in artificial intelligence in skin cancer. Clin Dermatol 2024:S0738-081X(24)00181-0. doi: 10.1016/j.clindermatol.2024.09.012.
- Kränke T, Tripolt-Droschl K, Röd L, Hofmann‑Wellenhof R, Koppitz M, Tripolt M. New AI-algorithms on smartphones to detect skin cancer in a clinical setting-A validation study. PLoS One 2023;18(2):e0280670. doi: 10.1371/journal.pone.0280670.
Response to ‘Melanoma imaging and diagnosis: What does the future hold?’
We read with interest the article, ‘Melanoma imaging and diagnosis: What does the future hold?’, by von Schuckmann et al.1 We concur that this technology might prove to be useful; however, is there a risk of harm? Our concerns are encapsulated in the title. Is ‘imaging’ a safe response to a lesion suspected of being a melanoma?
The paper makes the point that detecting changing or ‘unstable’ lesions can be problematic. What is meant by ‘unstable’ is not recorded. It has long been known that benign melanocytic naevi are dynamic and change over time.2 Conversely, Argenziano et al demonstrated over a decade ago that melanomas, even when invasive or indeed thick, do not necessarily change in appearance.3 In the study by Guitera et al, more melanomas were diagnosed in years 2–4 of monitoring a high-risk patient population than in years 0–2.4
The point was made that reliance on dermoscopic change shifted melanoma diagnosis to later time points.4
von Schuckmann et al assert that ‘The benefits of performing biopsies for suspicious pigmented lesions must be balanced with the potential to cause harm.’1 All involved in melanoma management would agree. This must beg the question of ‘what are the risks of taking images rather than biopsies of suspect lesions?’.
The authors state ‘Sequential photographs have been shown to increase the sensitivity and specificity of diagnosing melanomas.’1 This might be true of specificity (ie fewer benign lesions excised), but can it be true of sensitivity? Regardless of diagnostic technique, we never know how many melanomas are missed during a skin examination. Multiple studies of the use of short-term digital dermoscopic imaging reveal invasive and even thick melanomas belatedly excised after prolonged periods of imaging.3–5 The paper by von Schuckmann et al does not reference research showing that imaging of any kind results in decreased rates of metastasis or death.1
As Goldsmith pointed out, the cost savings from preventing metastasis or death from melanoma far outweigh the financial impact of any number of benign biopsies.6 Serial imaging is extensively documented to routinely result in avoidable delays in the diagnosis and hence treatment of invasive melanoma.5
Given the recent work by Xiong et al,7 who showed that a three-month delay in wide excision of stage 1 melanoma (invasive but <1 mm Breslow thickness) results in increased melanoma-specific mortality, it might well be that serial imaging will result in poorer overall morbidity and mortality than biopsy on suspicion. The clinical, financial and medico‑legal ramifications of delaying excision of suspect lesions through imaging in lieu of excision should be considered carefully.
Studies of short-term digital dermoscopic imaging reveal in situ : invasive ratios ranging from 0.59 to 2.88 from a combined total of 1755 melanomas, with up to 8.2% of the monitored melanomas being >1-mm thick.5 These in situ : invasive ratios are no better or indeed worse than those obtained within general practice, as described in studies by Hay et al8 (1.85:1)and Green et al (2.88:1).9 In contrast, where suspect lesions were removed on suspicion without any form of monitoring, the in situ : invasive ratio was 4.59:1.6 These outcomes cannot be directly compared as the patient groups and practitioner training are varied. However, they must raise doubts regarding the use and safety of serial imaging, and reinforce the need for prospective, randomised controlled trials.
von Schuckmann et al state ‘Early diagnosis is arguably the most important prognostic factor for melanoma survival’. We would agree entirely, but the currently available data do not support that sequential digital dermoscopic imaging (SDDI; with or without artificial intelligence) can produce this outcome. In fact, given the above in situ-to-invasive ratios and delayed diagnosis of thick melanomas seen with SDDI, the obverse is quite possibly true.
A recent Cochrane review by Johansson et al concluded that there was little to support population-based screening to reduce melanoma morbidity or mortality.10 The last 40 years have seen a great increase in melanoma overdiagnosis.11 An increased incidence of thin in-situ melanomas has not been offset by a decrease in thick (>4 mm) melanomas. A study by Olsen et al showed that incidence of thick (nodular) melanoma had increased at a faster rate than thin melanomas in their study population.12 We would ask the question, will SDDI, which is documented to frequently result in avoidable delays in melanoma diagnosis, merely replicate or even exacerbate these outcomes?
Given the costs of SDDI development and then, of patient access, would this money be better spent elsewhere in the fight against melanoma? Would the resources directed to these technologies and their associated access costs be better spent on an intervention we know to be useful? As demonstrated by Gordon et al, prevention has a far greater chance of reducing melanoma and indeed all solar-related skin cancer health impacts than early detection.13 This is especially so if we add the risks of delayed treatment.
Authors
Miranda Wallace BBioMedSc, MBBS, MS, Clinical Associate Lecturer, School of Medical Sciences, Faculty of Medicine and Health, The University of Sydney, Sydney, NSW; Dermatology Senior House Officer, Department of Dermatology, Mater Hospital, Brisbane, Qld
Jim Muir MBBS, FACD, Head of Department, Department of Dermatology, Mater Hospital, Brisbane, Qld; Dermatologist, South East Dermatology, Brisbane, Qld; Associate Professor, Dermatology, School of Medicine, The University of Queensland, Brisbane, Qld
Competing interests: None.
References
- von Schuckmann L, Banney L, Soyer HP. Melanoma imaging and diagnosis: What does the future hold? Aust J Gen Pract 2024;53(9):633–34. doi: 10.31128/AJGP-02-24-7156.
- Banky JP, Kelly JW, English DR, Yeatman JM, Dowling JP. Incidence of new and changed nevi and melanomas detected using baseline images and dermoscopy in patients at high risk for melanoma. Arch Dermatol 2005;141(8):998–1006. doi: 10.1001/archderm.141.8.998.
- Argenziano G, Kittler H, Ferrara G, et al. Slow‑growing melanoma: A dermoscopy follow-up study. Br J Dermatol 2010;162(2):267–73. doi: 10.1111/j.1365-2133.2009.09416.x.
- Guitera P, Menzies SW, Coates E, et al. Efficiency of detecting new primary melanoma among individuals treated in a high-risk clinic for skin surveillance. JAMA Dermatol 2021;157(5):521–30. doi: 10.1001/jamadermatol.2020.5651.
- Brown H, De’Ambrosis B, Yong-Gee S, Griffin A, Muir J. Melanoma diagnosis at a specialist dermatology practice without the use of photographic surveillance. Australas J Dermatol 2023;64(2):234–41. doi: 10.1111/ajd.14008.
- Goldsmith SM. Cost analysis suggests overemphasis on biopsy rate for melanoma diagnosis. J Am Acad Dermatol 2013;68(3):517–19. doi: 10.1016/j.jaad.2012.10.054.
- Xiong DD, Barriera-Silvestrini P, Knackstedt TJ. Delays in the surgical treatment of melanoma are associated with worsened overall and melanoma-specific mortality: A population-based analysis. J Am Acad Dermatol 2022;87(4):807–14. doi: 10.1016/j.jaad.2022.06.1190.
- Hay J, Keir J, Jimenez Balcells C, et al. Characteristics, treatment and outcomes of 589 melanoma patients documented by 27 general practitioners on the Skin Cancer Audit Research Database. Australas J Dermatol 2022;63(2):204–12. doi: 10.1111/ajd.13843.
- Green AC, Pandeya N, Morton S, Simonidis J, Whiteman DC. Early detection of melanoma in specialised primary care practice in Australia. Cancer Epidemiol 2021;70:101872. doi: 10.1016/j.canep.2020.101872.
- Johansson M, Brodersen J, Gøtzsche PC, Jørgensen KJ. Screening for reducing morbidity and mortality in malignant melanoma. Cochrane Database Syst Rev 2019;6(6):CD012352. doi: 10.1002/14651858.CD012352.pub2.
- Lindsay D, Bell KJL, Olsen CM, Whiteman DC, Pathirana T, Collins LG. Estimating the magnitude and healthcare costs of melanoma in situ and thin invasive melanoma overdiagnosis in Australia. Br J Dermatol 2024;ljae296. doi: 10.1093/bjd/ljae296. Epub ahead of print.
- Olsen CM, Pandeya N, Whiteman DC. The rise in thick melanomas: Can early detection reduce the burden? Br J Dermatol 2023;188(5):687–88. doi: 10.1093/bjd/ljad034.
- Gordon LG, Leung W, Johns R, et al. Estimated healthcare costs of melanoma and keratinocyte skin cancers in Australia and Aotearoa New Zealand in 2021. Int J Environ Res Public Health 2022;19(6):19. doi: 10.3390/ijerph19063178.
An update to the article, Restless legs syndrome, published in the AJGP September 2023 issue
We would like to issue a correction to our article published in the September 2023 issue of the AJGP (www1.racgp.org.au/ajgp/2023/september/restless-legs-syndrome).1 In Figure 1, dopamine agonists were incorrectly described as a treatment option for intermittent restless legs syndrome (RLS). This should have been low potency opioids (codeine, tramadol). Figure 1 has been updated accordingly and the role of low potency opioids for intermittent RLS described in the text. The following additional text belongs at the end of the first paragraph in the ‘Pharmacological management’ section of the text: ‘Lastly, low potency opioids such as codeine (30–90 mg) or tramadol (50–100 mg) can be effective for intermittent RLS but may have side effects including nausea and constipation.51’
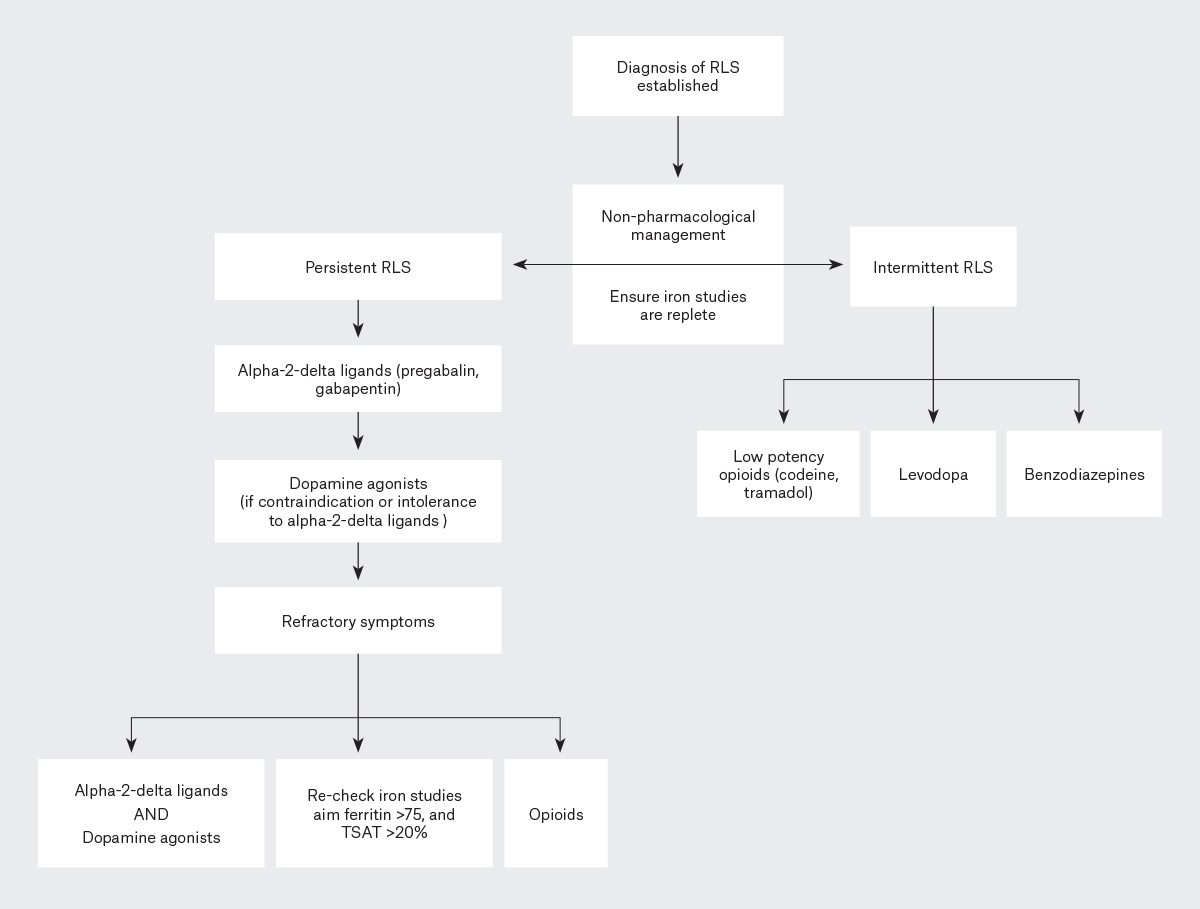 Figure 1. Treatment algorithm for the management of intermittent and persistent restless legs syndrome (RLS).
Figure 1. Treatment algorithm for the management of intermittent and persistent restless legs syndrome (RLS).
TSAT, transferrin saturation.
Furthermore, given the now well-recognised problem of augmentation with the use of dopaminergic medications for RLS, we have further altered the treatment of persistent RLS in Figure 1 to be clear that alpha-2-delta ligands, where not contraindicated or poorly tolerated, should be first-line therapy, as described in the text and reflected in current international RLS treatment algorithms.2,3 The following changes have been made to Figure 1:
- in the ‘Intermittent RLS’ pathway, ‘Dopamine agonists’ has been changed to ‘Low potency opioids (codeine, tramadol)’
- in the ‘Intermittent RLS’ pathway, the spelling of ‘Levadopa’ has been changed to ‘Levodopa’
- in the ‘Persistent RLS’ pathway, the diagram to reflect ‘Alpha-2-delta ligands (pregabalin, gabapentin)’ has been changed to be first-line therapy and ‘Dopamine agonists (if contraindication or intolerance to alpha-2-delta ligands)’ is now second-line therapy.
Authors
Budhima Nanayakkara PhB (Hons 1), MBBS (Hons 1), FRACP, Associate Professor in Medicine, School of Rural Medicine, Charles Sturt University, Orange, NSW; Director of Prevocational Education and Training, Orange Health Service, Orange, NSW; Staff Specialist Respiratory and Sleep Physician, Orange Health Service, Orange, NSW
James Di Michiel MBBS, FRACP, Respiratory and Sleep Physician, Woolcock Institute of Medical Research, Sydney, NSW; Staff Specialist Respiratory and Sleep Physician, Canterbury Hospital, Sydney, NSW; Visiting Medical Officer, Griffith Base Hospital, Griffith, NSW
Brendon J Yee MBChB, FRACP, PhD, Professor, Central Clinical School, Faculty of Medicine and Health, The University of Sydney, Sydney, NSW; Senior Staff Specialist Respiratory and Sleep Physician, Department of Respiratory and Sleep Medicine, Royal Prince Alfred Hospital, Sydney, NSW; Senior Researcher/Clinician Fellow, Centre for Sleep and Chronobiology, Woolcock Institute of Medical Research, Sydney, NSW
Competing interests: BN has received honoraria for lectures and presentations from GSK, AstraZeneca, Chiesi, Boehringer Ingelheim and SomnMed. JDM and BJL have no competing interests to declare.
References
- Nanayakkara B, Di Michiel J, Yee BJ. Restless legs syndrome. Aust J Gen Pract 2023;52(9):615–21. doi: 10.31128/AJGP-02-23-6722.
- Allen RP, Picchietti DL, Auerbach M, et al; International Restless Legs Syndrome Study Group (IRLSSG). Evidence-based and consensus clinical practice guidelines for the iron treatment of restless legs syndrome/Willis-Ekbom disease in adults and children: An IRLSSG task force report. Sleep Med 2018;41:27–44. doi: 10.1016/j.sleep.2017.11.1126.
- Garcia-Borreguero D, Silber MH, Winkelman JW, et al. Guidelines for the first-line treatment of restless legs syndrome/Willis-Ekbom disease, prevention and treatment of dopaminergic augmentation: A combined task force of the IRLSSG, EURLSSG, and the RLS-foundation. Sleep Med 2016;21:1–11. doi: 10.1016/j.sleep.2016.01.017.
Response to ‘A pragmatic primary care approach to the patient with significant solar damage’
We read with interest the article by Charles Ayesa, ‘A pragmatic primary care approach to the patient with significant solar damage’, which was published in the AJGP August 2024 issue.1 The article addresses the common issue of excluding keratinocyte malignancy from actinic keratosis by conducting a partial biopsy. It advises that a punch biopsy is ‘favoured over shave biopsy for hyperkeratotic lesions, as the base of the lesion needs examination by the pathologist for accurate diagnosis’. It is unclear why an appropriate shave procedure, where the depth and width of tissue harvested is at the discretion of the operator, would prevent the pathologist from examining the base of the lesion. Indeed, the reference used to support this erroneous claim states ‘small punch biopsies or superficial shave biopsies can lead to diagnostic challenges’.2
If malignancy is suspected, a shave should be done to the level of the deeper dermis. With most lesions where a clinician considers solar keratosis or keratinocyte malignancy possible, a competent shave will harvest the entire width of the lesion. The only reason the base of the lesion could not be examined is if the sample was very superficial or the tumour penetrated into the sub-cutis; the former is an error of technique, the latter one of clinical assessment. A shave into deep dermis would, of course, confirm an invasive lesion and exclude solar keratosis.
If solar keratosis is the diagnosis after a shave, then typically no further treatment is needed. A punch biopsy, in contrast, will leave residual lesion, often requiring further intervention. In support of this is the study by Ilyas et al, which showed that after shave biopsy of squamous cell carcinoma in immunocompetent patients, only 31 of 117 lesions showed residual squamous cell carcinoma (SCC) on definitive excision.3
Any partial biopsy risks misdiagnosis or underdiagnosis compared to complete excision. A study by Russell et al demonstrated many years ago that punch and shave biopsies were equivalently accurate in the diagnosis of basal cell carcinoma.4 The larger the sample submitted, the more likely a partial biopsy will accurately diagnose a lesion. Punch biopsies are far from optimal in this regard. In another study, Westers-Attema et al showed that 3-mm punch biopsies of SCCs routinely missed high-risk features such as depth of invasion, perineural invasion and poor differentiation.5
Shave procedures are quick, same-day procedures, allowing multiple lesions to be sampled, requiring minimal equipment, little morbidity, no return visit for suture removal and excellent cosmesis. We would argue that given good lesion selection and technique, shave biopsies represent a much more ‘pragmatic’ approach to the management of patients with significant solar damage compared with reliance on punch biopsies.
Authors
Miranda Wallace BBioMedSc, MBBS, MS, Clinical Associate Lecturer, School of Medical Sciences, Faculty of Medicine and Health, The University of Sydney, Sydney, NSW; Dermatology Senior House Officer, Department of Dermatology, Mater Hospital, Brisbane, Qld
Jim Muir MBBS, FACD, Head of Department, Department of Dermatology, Mater Hospital, Brisbane, Qld; Dermatologist, South East Dermatology, Brisbane, Qld; Associate Professor, Dermatology, School of Medicine, The University of Queensland, Brisbane, Qld
Competing interests: None.
References
- Ayesa C. A pragmatic primary care approach to the patient with significant solar damage. Aust J Gen Pract 2024;53(8):547–53. doi: 10.31128/AJGP-02-24-7159.
- Tan KB, Tan SH, Aw DC, et al. Simulators of squamous cell carcinoma of the skin: Diagnostic challenges on small biopsies and clinicopathological correlation. J Skin Cancer 2013;2013:752864. doi: 10.1155/2013/752864.
- Ilyas M, Zhang N, Sharma A. Residual squamous cell carcinoma after shave biopsy in solid organ transplant recipients. Dermatol Surg 2018;44(3):370–74. doi: 10.1097/DSS.0000000000001340.
- Russell EB, Carrington PR, Smoller BR. Basal cell carcinoma: A comparison of shave biopsy versus punch biopsy techniques in subtype diagnosis. J Am Acad Dermatol 1999;41(1):69–71. doi: 10.1016/S0190-9622(99)70409-3.
- Westers-Attema A, Joosten VM, Roozeboom MH, et al. Correlation between histological findings on punch biopsy specimens and subsequent excision specimens in cutaneous squamous cell carcinoma. Acta Derm Venereol 2015;95(2):181–85. doi: 10.2340/00015555-1826.
Reply
Thank you for your interest in my article, ‘A pragmatic primary care approach to the patient with significant solar damage’, which was published in the AJGP August 2024 issue1 and raising some valid points. It needs to be considered, however, that this article is aimed at the broader general practice audience, including doctors who are less experienced in skin cancer diagnosis and management. The nuances of examining a hyperkeratotic lesion with a thickened, indurated base or nodular architecture requires experience to guide whether a shave or punch biopsy is more appropriate. I regard the shave biopsy technique as a more advanced-level technique. The method of the technique is not difficult to learn and perform; however, the decision to use it and the skill necessary to modify the depth according to site and lesion requires a more advanced level of understanding of the lesion needing to be biopsied than performing a partial punch biopsy. I have observed this with my teaching of medical students, general practitioner (GP) registrars and GP consultants.
I agree that appropriate use of a shave biopsy can be used to diagnose hyperkeratotic lesions that might be actinic keratoses or potentially intraepidermal or invasive squamous cell carcinoma (SCC), provided the shave is deep enough. I also agree that a small punch biopsy of a larger lesion might lead to sampling error in a heterogenous lesion. This is where more advanced dermatoscopic skills might guide the most appropriate area(s) to biopsy. Submitting a sample large enough to be representative of the lesion is always the aim, and a larger specimen will usually be less likely to lead to sample error and misdiagnosis. I have conferred with a couple of dermatopathologists, and they agree that both shave and punch biopsies can give comparable diagnostic accuracy for a basal cell carcinoma (BCC; subtype dependent) and a punch biopsy might miss critical features in SCC diagnosis. Shave biopsies are an excellent tool in experienced hands. Nevertheless, we must be cautious not to over generalise their applicability in all patient encounters. I employ the use of shave biopsies (partial and complete removal) judiciously and often in my own personal practice; commonly, in combination with serial curettage and electrofulguration to treat superficial keratinocyte cancers. I agree it is a quick technique allowing multiple lesions to be sampled and treated in the same visit, typically requiring only a phone call for the result. The issue, as you correctly pointed out, is that good lesion selection and technique are paramount, and a less-experienced colleague might be better choosing a punch biopsy over a shave biopsy when dealing with a lesion that could be invasive SCC. Time and experience to develop this expertise is required, preferentially under guidance through experienced colleagues/mentors and from formal teaching processes.
There are some circumstances where a punch biopsy might be preferred over a shave biopsy. For example, a shave on the hand of an elderly person with chronic sun damaged skin and atrophic dermis will possibly result in a full-thickness defect with subsequent prolonged healing time. It should also be noted that although shave biopsies can produce good cosmetic results, areas prone to hypertrophic scarring (eg chest and shoulders) are unlikely to have a good cosmetic result when compared to the use of a punch biopsy. Shave biopsies on the face also result in colour and contour deformities, especially in areas of glabrous skin of high-density sebaceous units such as the nose and forehead. Generalising that ‘excellent cosmesis’ occurs in general is not advisable. Patient morbidity concerns also should be considered as patients, in my experience, usually complain more about the healing process of a shave biopsy over a punch biopsy and their aftercare is considerably more complicated. A thicker, nodular lesion biopsied with a shave, which transects the lesion, might not give enough information to the pathologist and might result in an ‘atypical squamous proliferation’ diagnosis or similar, necessitating a judgement call to be made – usually an excision. For a larger exophytic, tender, fast-growing lesion, it would still be better to perform a punch biopsy larger enough (eg 6 or 8 mm) to obtain a representative sample and ascertain the depth of the lesion. The other benefit in this scenario is that the tumour will effectively be debulked, which might lead to a smaller definitive excision later; though one would argue it might be better to proceed straight to definitive excision. A large punch might also be used as a means of definitive excision of smaller lesions.
The pros and cons of every case needs to be considered, as does the potential delay in definitive management by performing a partial biopsy (either shave, punch or incisional) first, which is dependent on the diagnostic expertise of the doctor and the availability of both the patient and the doctor. The shave biopsy and punch biopsy are different tools available and each needs to be considered correctly in each circumstance to ensure what is in the best interests of our patients to achieve the best outcomes.
Author
Charles Ayesa MBBS (Hons), BMedSci, FRACGP, FSCCA, College Censor, Skin Cancer College Australasia, Brisbane, Qld; Clinical Senior Lecturer, The University of Queensland, Faculty of Health Sciences Primary Care Unit, Brisbane, Qld; Honorary Senior Lecturer, Macquarie University, MQ Health, Sydney, NSW; General Practitioner, SkinSense Clinic, Sydney, NSW; General Practitioner, Icon Cancer Centre, Sydney, NSW
Competing interests: None.
References
1. Ayesa C. A pragmatic primary care approach to the patient with significant solar damage. Aust J Gen Pract 2024;53(8):547–53. doi: 10.31128/AJGP-02-24-7159.