Familial hypercholesterolaemia (FH) is a co-dominantly inherited disorder of low-density lipoprotein cholesterol (LDL-C) metabolism characterised by elevated levels from birth that can progress to premature atherosclerotic coronary artery disease if left untreated.1–3
Early detection and treatment are clinically and economically effective, but detection rates worldwide remain low at approximately 1%.4 Despite increased attempts to improve awareness, FH continues to remain underdiagnosed and undertreated.5 This is mainly due to a lack of effective screening strategies for potential index cases in the community6 followed by appropriate management of the condition and subsequent cascade testing of close relatives. Some of the largest gaps in FH detection exist among patients attending primary healthcare settings.5,7,8
Because of their central position in the healthcare system in Australia coupled with easy access to primary care services, general practitioners (GPs) are well placed to play a larger part in improving FH detection and management.9 A shared model of care between non-GP specialists and GPs is recognised as offering the optimal approach for future management, especially for patients at low-to-intermediate risk.6
The potential economic benefits of such an approach are becoming more attractive, with increasing evidence for earlier, more comprehensive and finely tuned management approaches.4,9,10 However, health economic evaluation of such an increased general practice role is still lacking. Our recent study indicated that detection and management of FH in general practice is feasible and scalable on a national level.11 Using a two-stage approach combining electronic medical record screening and subsequent clinical assessment, 147 patients with phenotypic FH were identified, with further evidence of lowered LDL-C levels from GP management. This provides an opportunity and imperative to estimate the cost effectiveness of such an approach.
The present study examines the following. First, we describe the management pathways and associated costs for patients with FH in the general practice setting. Second, we aim to model the cost effectiveness of GP identification and management of patients with FH, seeking to avert costly downstream management in the tertiary setting due to delayed diagnosis and management.
Methods
Study design
The study protocol has been published previously,12 as have results from screening and management phases.11 Fifteen general practices from five Australian states participated in the study. Medical records from 232,139 patients were screened using a validated data extraction tool, TARB-Ex, to identify patients at high risk.13 Those patients were recalled for clinical assessment and medical history review by GPs. A phenotypic diagnosis of FH was made for those with a Dutch Lipid Clinic Network Criteria (DLCNC) score6 of ≥6. Patients whose FH was assessed as low-to-intermediate complexity were managed by a GP/practice nurse (PN), with a shared-care approach with a lipid specialist available if required and for patients with complex conditions. Such patients with complex conditions met the criteria for Chronic Disease Management plans,14 which are listed on the Medicare Benefits Schedule (MBS; items 721 and 723). This funding helped ensure sustainability in follow-up care.
Cost of GP management of FH
Data on the following information were collected: consultation category (MBS item code),15 proportion of consultation spent addressing FH, current medication, new (if any) medication prescribed, blood tests ordered, imaging ordered, other testing ordered, referrals made. Data collected were linked to costs provided by government sources (Table 1). All costings are presented in 2018 Australian dollars.
| Table 1. Cost categories and sources |
| Type of cost |
Source |
| Consultation category |
Consultation type (MBS) × proportion of consultation related to FH |
| Medication |
Pharmaceutical Benefits Scheme (Dispensed Price for Maximum Quantity) cost of prescribed cholesterol-lowering medications. Medication type and dosage were reported from collaborating centres, and patients were assumed to purchase 30 tablets per 30 days for the study duration unless dose and/or medication were changed. |
| Pathology, imaging |
Item cost (MBS). We assumed 100% of the item cost related to FH (eg blood lipids). |
| FH, familial hypercholesterolaemia; MBS, Medicare Benefits Schedule |
Cost implications for the healthcare system
We evaluated the costs of management of FH against life-years gained using a life table model. We obtained male and female life tables for Australia from the UN World Population Prospect 2019 data16 and simulated two scenarios, one in which the Australian population with FH are not managed for FH, and another in which FH is managed in primary care. These scenarios were compared against the life table for the general population.
We obtained standardised mortality ratios for these scenarios from the Simon Broome Register of Familial Hyperlipidaemia,17 which were applied by adjusting the probability of dying by the standardised mortality ratio between birth and age 60 years. The major assumptions for the data are referenced and explained subsequently. The complete life table can be found in Appendix 1.
Ethical approval
The study approved by The University of Notre Dame Australia Human Research Ethics Committee Protocol ID: 016067F.
Results
Management pathways for identified patients with FH
From an original pool of 67,932 patients with LDL-C measurements, 133 patients with baseline consultation and lipid information consented to participate in the study (Appendix 2). Of these baseline consultations, the majority (82%) were billed as a regular consultation under the MBS item 23, 36 or 44,15 whereas fewer (13%) were billed as a Chronic Disease Management item (MBS item 721, 723 or 732). 14 However, this proportion increased over the follow-up period, with 23% of all follow-up consultations billed as a Chronic Disease Management item and 77% billed as a regular visit.
The costs of managing FH in a general practice setting
Table 2 presents the empirical findings for costs associated with general practice management. For both consultation and medications, median costs did not rise substantially from baseline to follow-up. When considering the findings over the first year of the study, the median cost of management was $275, inclusive of consultations, medications and lipid tests, with the interquartile range between $225 and $482 (Figure 1).
| Table 2. Costs of consultations and medications at baseline and follow-up consultations |
| Unit costs |
Value |
Range (25%–75%) |
| MBS item 23 – Level B consultations |
$39.10 |
– |
| MBS item 36 – Level C consultations |
$75.75 |
– |
| MBS item 44 – Level D consultations |
$111.50 |
– |
| MBS item 721 – GP Management Plan preparation |
$150.10 |
– |
| MBS item 723 – Coordination of Team Care Arrangement |
$119.95 |
– |
| MBS item 732 – Review of management plan or team care |
$74.95 |
– |
| Consultations and medications estimates, mode and median |
| Consultations |
| Most common MBS item type |
23 |
|
| Median consultation cost at baseline visit |
$38.75 |
$38.75–$75.05 |
| Median consultation cost at first follow-up |
$38.75 |
$38.75–$75.05 |
| Median consultation cost at second follow-up |
$38.75 |
$38.75–$75.27 |
| Median consultation cost at third follow-up |
$38.75 |
$37.53–$88.00 |
| Medications |
| Most common medication prescribed |
Rosuvastatin |
|
| Median cost of medication at baseline visit |
$16.56 |
$15.19–$17.55 |
| Median cost of medication at first follow-up |
$17.55 |
$15.19–$34.07 |
| Median cost of medication at second follow-up |
$17.55 |
$15.19–$34.31 |
| Median cost of medication at third follow-up |
$17.55 |
$16.22–$35.55 |
Note: Medication costs refer to Dispensed Price for Maximum Quantity (DPMQ).
MBS, Medicare Benefits Schedule |
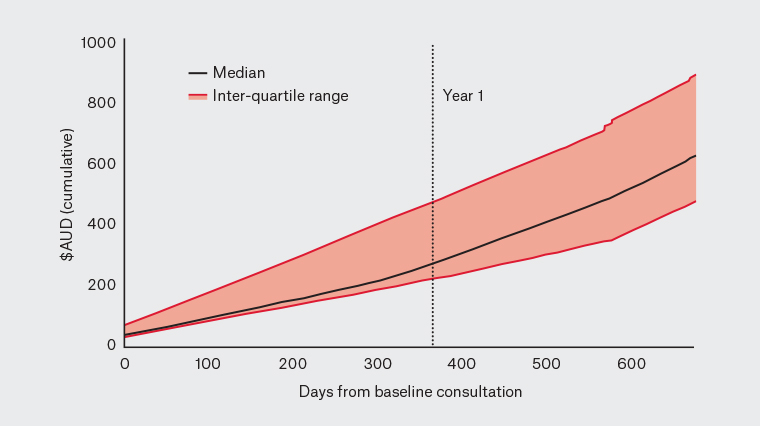
Figure 1. Cumulative costs per patient from day 0 (baseline consultation) until final follow‑up (median and interquartile range)
Cost implications for the healthcare system
Table 3 illustrates the implications of GP-led FH management on healthcare costs of treating major cardiac events. Briefly, the table models the cost implications to the healthcare system of managing 45,974 individuals, the estimated number of individuals with FH in the community based on prevalence estimates from the literature2 and population figures published by the Australian Bureau of Statistics.18,19 Managing patients with statins over this period yielded a total number of life-years gained of 248,954, representing an average gain of five life-years per patient with FH in the community. Managing all community members until the age of 60 years, where the benefit of statin therapy is assumed to diminish, cost $759 million ($275 per person per annum), representing a cost per life-year gained of $3047. Additional details can be found in Appendix 1.
| Table 3. Modelling the cost implications of general practice–based FH management and downstream major cardiac events averted |
| Statement |
Value |
Notes |
| Proportion of adults in the community with FH |
0.0026 |
Unadjusted probable prevalence2 |
| Total number of adults in the community with FH |
45,974.57 |
Proportion of population aged ≥25 years, 2016 Census14 × 0.0026 × Population of Australia, June 202118 |
| Person years lived from birth in untreated FH scenario |
3,296,918.55 |
Lifetable calculation, refer to Appendix 1 (available online only) |
| Person years lived from birth in managed FH scenario |
3,545,872.96 |
Lifetable calculation, refer to Appendix 1 (available online only) |
| Life-years gained (from management) |
248,954.41 |
Difference between
person-years lived |
| Yearly cost of managing FH |
$275 |
Empirical finding
(see Results section) |
| Cost of managing FH from ages 0–60 years |
$16,500 |
Yearly cost × 60 |
| Cost of managing entire community with FH over lifetime |
$758,580,463.26 |
Lifetime cost × adults in community with FH |
| Cost per life-year gained |
$3,047.07 |
|
| FH, familial hypercholesterolaemia |
Discussion
The current study was undertaken in the real-world clinical setting of Australian general practice using existing practice infrastructure. Our pragmatic approach improved the care of patients with FH, as reflected by the higher percentage of follow-up consultations under Chronic Disease Management items. We also found that this GP management approach was cost effective, with a cost per life-year gained of $3047.
Change in management pathway for identified patients with FH
Our earlier research showed a pragmatic approach involving electronic health record screening and follow-up clinical consultations was feasible for identifying and better managing patients with FH in the primary healthcare setting.11 We extended the study to investigate the impact of this approach on the management pathways for identified patients with FH and potential cost benefits.
There was a moderate increase in the proportion of patients with FH billed for Chronic Disease Management items on subsequent consultations, reflecting an improvement in targeted GP management. While these results are encouraging, they only account for 23% of all billed follow-up consultations, with most patients continuing to be billed for regular consultations. It is possible that lack of awareness of FH among treating GPs in addition to their lack of familiarity with FH meeting all the criteria for Chronic Disease Management plans could explain the lower-than-expected uptake of these specific item numbers.14
The potential future sustainability of increased GP involvement in the detection and management of FH will be highly dependent on such specific funding addressing the additional workload GPs incur in managing FH in primary care combined with non-GP specialist and allied health involvement through specific Team Care Arrangements. The use of Chronic Disease Management plans for patients with FH is likely to increase in the future with minimum six-monthly follow-up checks.
Cost analysis
While it might appear intuitively logical that primary care services are inherently less expensive than tertiary hospital-level care, robust health economic evaluations of FH management costs and infrastructure capacity in general practice to undertake an expanded FH role remain elusive. This evaluation focuses on the cost implications of the method of care that we employed. Costs of screening tests using data extraction tools for patients at high risk of conditions such as FH have been shown to be much less costly and much faster than manual medical record review.13 The net cost impact of saving life-years is reasonably affordable.
The majority of patients recalled for clinical review were billed for regular general practice consultations on their initial diagnostic consultation. Once a FH diagnosis is confirmed, the burden on practices reduces, and patients with FH became eligible for Chronic Disease Management items for their ongoing chronic disease care.14 There is potential for further cost efficiency in FH management over time, with patient reviews incorporated into other clinic visits, and FH only a proportion of the total cost.
The cost implication result for treatment of patients with FH in primary care – $3047 per life-year gained – compares favourably with known cost-effectiveness thresholds internationally. For instance, health interventions costing up to 30,000 euros per life-year gained are considered cost effective for the purposes of public subsidy.20 A recent Australian study indicated that an attractive cost-effectiveness threshold for Australia can be up to $40,000,21 although it should be noted that this is measured in quality-adjusted life-years. Further investigation should examine the health economic impact of managing FH on cost effectiveness (eg Markov model) and productivity.4,22
Limitations
The present study has limitations. First, the findings are based on a relatively small sample of 133 patients with an FH diagnosis. Second, the duration of follow-up was relatively short. Some of the data collection took place in 2020 amidst the global COVID-19 pandemic, which likely affected study participation and follow-up. Third, the model of GP-based management of FH did not include potential costs for non-GP specialist co-management for cases that are complex. Fourth, other relevant economic impacts from life-years lost, averted downstream health expenditure and lost productivity were not considered in the analysis. Inclusion of these costs would arguably result in a stronger case for early detection and management of FH.
Further and larger studies on the diagnosis, treatment and management of FH in the primary care setting will be valuable for providing further evidence on the cost implications and cost effectiveness of the process. Our ‘real-world’ study is based on successful diagnosis and management of patients. Actual results ‘in the field’ could be less favourable because of factors such as undetected cases and patient compliance with management and medication, and future research should investigate these factors further.
Conclusion
The recent Integrated guidance for enhancing the care of familial hypercholesterolaemia in Australia highlights the central role of general practice in the continuity of care of all patients with FH and their families.10 The guidance encourages a more active role for GPs in ‘screening, diagnosis, supporting families, shared care with other specialities, managing cholesterol-lowering medication and multimorbidities’ while also contributing to the implementation of context-specific models of care for FH.10 Our cost analysis supports screening and management of FH in general practice having the potential for substantial health benefits while requiring relatively modest investments from the health system. Most pathology testing in Australia is bulk billed with no out-of-pocket costs to the patient, while medications have minimal out-of-pocket costs because of subsidy from the Pharmaceutical Benefits Scheme. The high cost effectiveness of genetic cascade testing is known – verification in the general practice setting may be the next step.23
Implications for general practice
- Health economic evidence of GP-based management of FH is scarce.
- Early detection and management of FH in a general practice setting can be very cost effective.
- A GP-based approach costs $3047 per life-year gained.
- Chronic Disease Management plans are underused but cost efficient and will make health system expenditure sustainable.
- There is potential for GPs to have larger roles in FH management.